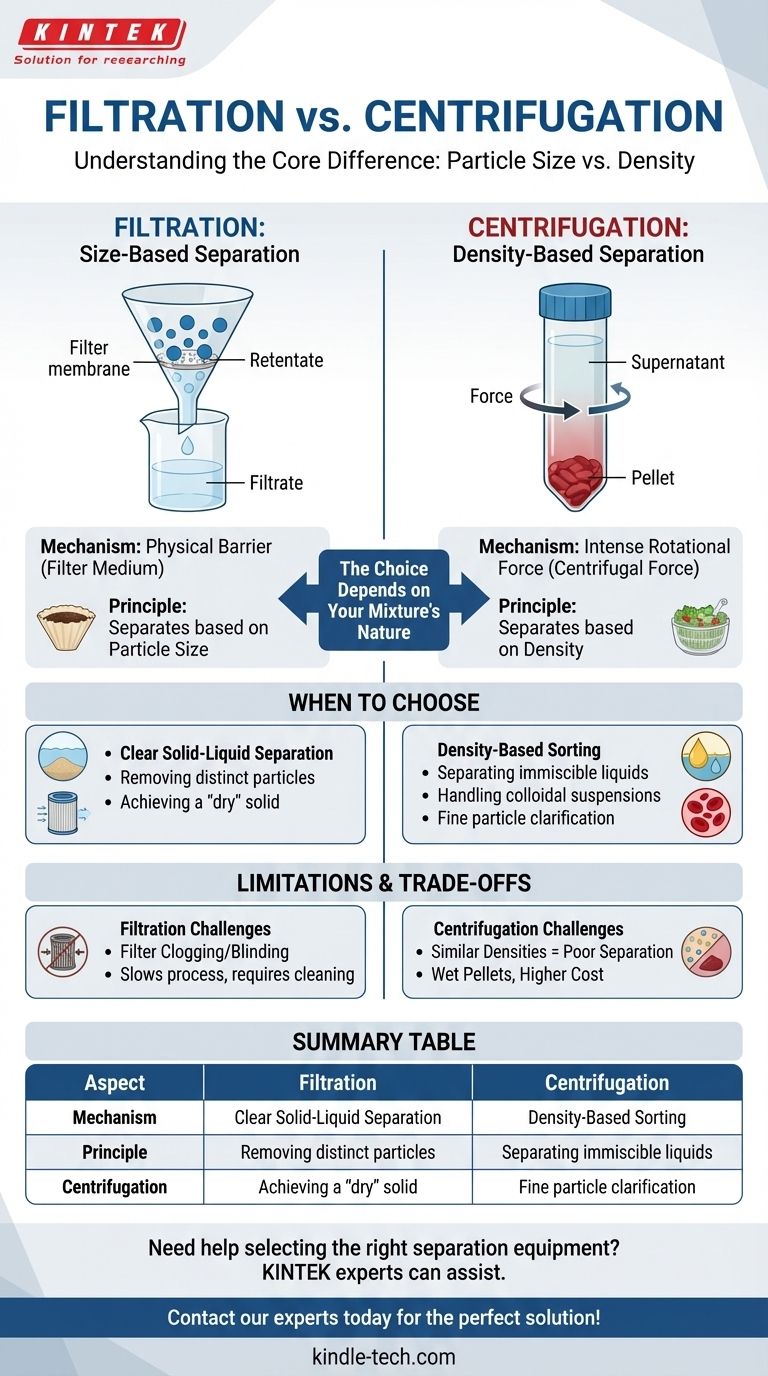
At its core, the difference is simple: Filtration separates materials based on particle size, while centrifugation separates them based on density. Filtration uses a physical barrier—a filter medium—to block particles larger than its pores, allowing only the fluid to pass through. In contrast, centrifugation uses intense rotational force to accelerate the natural process of sedimentation, causing denser components to move away from the center of rotation and less dense components to move toward it.
The choice between these two methods is not about which is "better," but about the fundamental nature of your mixture. Filtration is a mechanical sieve for separating solids from fluids based on size. Centrifugation is a force-based tool for sorting components by their density, making it effective for everything from fine particles in a liquid to different types of liquids themselves.

The Fundamental Principle: Size vs. Density
The effectiveness of each technique is rooted in a different physical property. Understanding this distinction is the key to selecting the right method for your specific separation task.
How Filtration Works: A Physical Barrier
Filtration is an intuitive, mechanical process. It works by passing a mixture through a porous medium.
The fluid and any dissolved substances pass through the pores, becoming the filtrate. Any suspended particles too large to fit through the pores are trapped on the surface of the medium, becoming the retentate.
Think of a simple coffee filter. Water (the fluid) passes through, but the ground coffee particles (the solids) are too large and get left behind. The process relies entirely on a size differential between the particles and the filter pores.
How Centrifugation Works: Amplified Gravity
Centrifugation does not use a physical barrier. Instead, it exploits differences in density by creating a powerful artificial gravitational field.
When a mixture is spun at high speed in a centrifuge, it experiences immense centrifugal force. This force causes denser components to sediment much faster than they would under normal gravity.
Denser particles or liquids are forced to the outer edge of the container, forming a compact layer or pellet. Less dense components are displaced and move toward the center, forming a liquid layer called the supernatant. A salad spinner works on a similar principle, using force to separate water from the denser lettuce.
When to Choose One Over the Other
The nature of your mixture and your end goal will dictate the correct choice. One method may be highly effective where the other fails completely.
Use Filtration for Clear Solid-Liquid Separation
Filtration is the ideal choice when you have well-defined solid particles suspended in a liquid or gas and your goal is to remove them.
This method excels at tasks like removing sand from water, purifying air with a HEPA filter, or collecting a precipitated chemical from a solution. The result is a clean fluid and a separated, often "dry," solid.
Use Centrifugation for Density-Based Sorting
Centrifugation is the superior tool when components are separated by density, not just size. This is critical for several common scenarios.
It is highly effective for separating two immiscible liquids (like oil and water) or for clarifying a liquid containing very fine particles that would quickly clog a filter. It is the standard method in biology for separating blood cells from plasma or organelles from cell lysate.
Handling Colloidal Suspensions and Emulsions
For mixtures where particles are extremely small (e.g., colloids, emulsions, or macromolecules), filtration is often useless. The particles are smaller than the pores of most conventional filters and will simply pass through.
In these cases, high-speed centrifugation (or ultracentrifugation) is the only viable method. The immense g-forces generated are capable of forcing even these tiny, low-mass particles to sediment based on their slight density differences.
Understanding the Trade-offs and Limitations
Neither technique is a perfect solution for all problems. Being aware of their inherent limitations is crucial for successful separation.
The Problem of Filter Clogging
The primary weakness of filtration is blinding or clogging. If the mixture has a high concentration of solids, or if the particles are gelatinous or compressible, they can quickly block the filter's pores.
This dramatically slows down or even halts the separation process, requiring filter replacement or cleaning, which adds time and cost.
The Limitations of Centrifugal Force
Centrifugation struggles when the components of a mixture have very similar densities. The separation will be slow and incomplete, even at high speeds.
Furthermore, the separated solid "pellet" is not dry. It remains saturated with the liquid supernatant, which may require a secondary washing or drying step if a pure solid is the goal.
Considerations of Scale and Cost
At a small laboratory scale, filtration can be extremely cheap and simple (e.g., filter paper and a funnel). However, scaling up filtration for industrial processes can involve complex and expensive equipment.
Centrifuges, on the other hand, represent a higher upfront capital cost. They require electricity, regular maintenance, and careful balancing to operate safely, making them a more significant investment than simple filtration setups.
Making the Right Choice for Your Mixture
Your decision depends entirely on the physical properties of your mixture and your desired outcome. Use these guidelines to make a clear choice.
- If your primary focus is removing visible, distinct solid particles from a liquid: Filtration is your most direct and often simplest method.
- If your primary focus is separating components with different densities (like cells, organelles, or immiscible liquids): Centrifugation is the correct tool, as size is less relevant than density.
- If your primary focus is separating extremely fine, sub-micron particles or macromolecules: High-speed centrifugation is necessary, as these particles would pass right through most filters.
- If your primary focus is achieving a "dry" solid in a single step: Filtration is generally superior, provided the particles are large enough to be retained.
By understanding this core difference between size-based and density-based separation, you can select the right tool to achieve a clean and efficient result for your specific goal.
Summary Table:
| Aspect | Filtration | Centrifugation |
|---|---|---|
| Separation Principle | Particle Size | Particle Density |
| Mechanism | Physical Barrier (Filter) | Centrifugal Force |
| Ideal For | Removing distinct solids from liquids | Separating immiscible liquids, cells, fine particles |
| Key Limitation | Filter Clogging | Similar Densities, Wet Pellets |
Need help selecting the right separation equipment for your laboratory?
Whether your process requires precise filtration media or high-performance centrifuges, KINTEK has the expertise and product range to support your specific application. Our lab equipment and consumables are designed to deliver reliable, efficient separation results.
Contact our experts today to discuss your needs and find the perfect solution!
Visual Guide
Related Products
- Laboratory Hydraulic Press Lab Pellet Press Machine for Glove Box
- Automatic Lab Cold Isostatic Press CIP Machine Cold Isostatic Pressing
- Electric Lab Cold Isostatic Press CIP Machine for Cold Isostatic Pressing
- Custom PTFE Teflon Parts Manufacturer for Centrifuge Tube Racks
- Automatic Laboratory Heat Press Machine
People Also Ask
- What are the environmental issues with diamond mining? Uncover the True Ecological and Human Cost
- Which heat treatment process is used to give steel a high resistance against wear? Achieve Maximum Durability with Case Hardening
- How much does a biochar production facility cost? From $100k to $10M+ for Your Project
- How plasma is created in sputtering? A Step-by-Step Guide to Ionization and Thin Film Deposition
- What units are used for heat capacity? A Guide to J/K, J/(kg·K), and J/(mol·K)
- What are the benefits of bio-oil pyrolysis? Turn Waste into Renewable Energy
- Why is deposition technology an amazing scientific advancement? Unlock Atomic-Level Material Engineering
- What temperature does THC evaporate in a vacuum? Master the Distillation Process



















