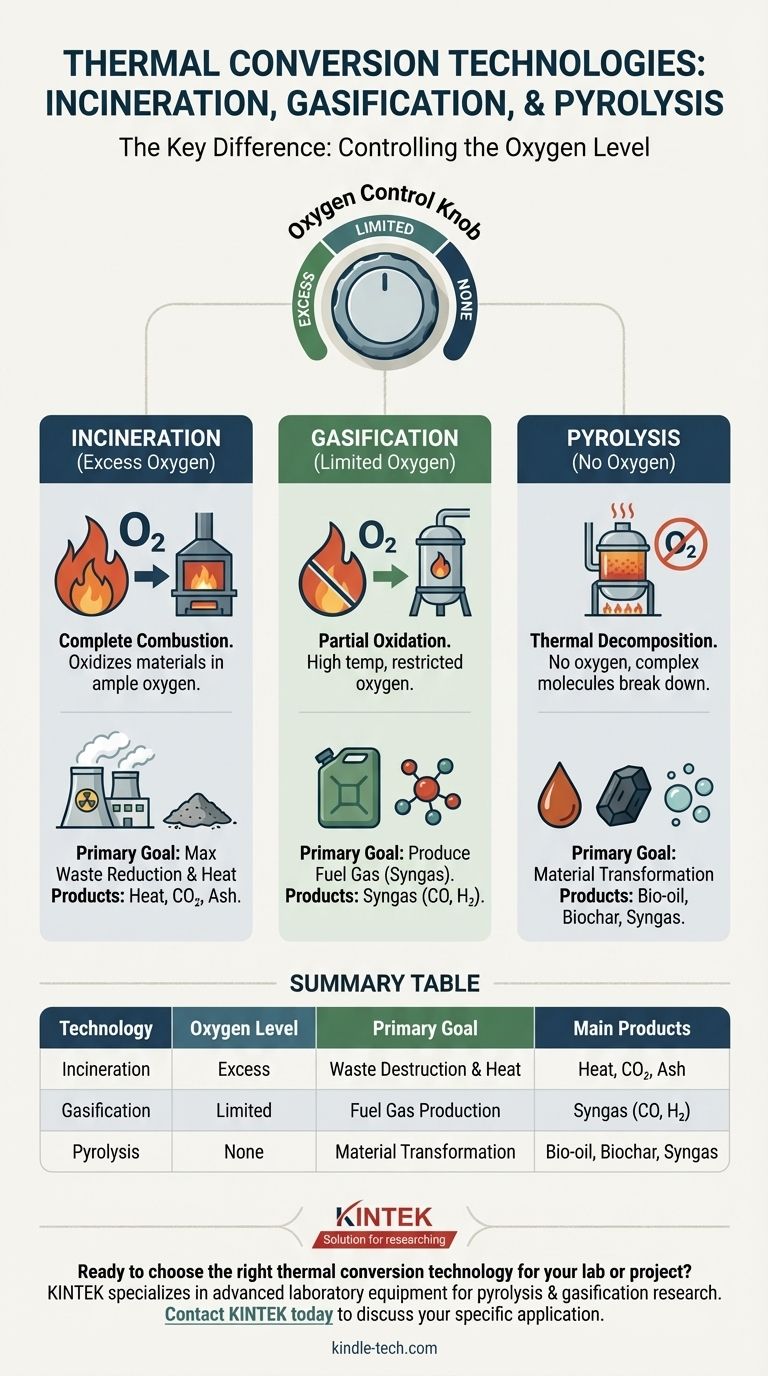
The fundamental difference between incineration, gasification, and pyrolysis lies in the amount of oxygen present during the process. Incineration involves complete combustion with an excess of oxygen, gasification uses a restricted amount of oxygen for partial combustion, and pyrolysis occurs in the complete absence of oxygen. This single variable dictates the chemical reactions, final products, and ultimate purpose of each technology.
The choice between these three thermal conversion methods is not about which is "best," but about the desired outcome. The amount of oxygen used acts as a control knob, determining whether the goal is to release energy as heat or to convert a material into valuable fuels and chemical products.

The Critical Role of Oxygen
Oxygen is the key reactant that determines the pathway of thermal conversion. By controlling its supply, we can steer the process from simple destruction to complex transformation.
Incineration: Complete Combustion
Incineration is the process of burning organic materials in an environment with ample oxygen. It is a form of complete oxidation.
The primary goal of incineration is to maximize heat release and achieve the greatest possible volume reduction of the initial material, such as municipal solid waste.
The main outputs are heat (used to create steam for electricity or heating), carbon dioxide (CO₂), water, and a solid residue known as ash.
Gasification: Partial Oxidation
Gasification exposes carbon-based materials to high temperatures (typically above 700°C) with a limited or "starved" supply of oxygen.
This prevents full combustion. Instead of just producing heat and CO₂, the process intentionally creates a mixture of combustible gases.
The primary product is synthesis gas, or syngas, which is a fuel composed mainly of carbon monoxide (CO) and hydrogen (H₂). This gas can then be used to generate electricity or as a building block for producing chemicals and liquid fuels.
Pyrolysis: Thermal Decomposition without Oxygen
Pyrolysis is the thermal decomposition of materials at high temperatures in the complete absence of oxygen.
Since there is no oxygen to react with, the material does not combust. Instead, the complex organic molecules break down into simpler, smaller molecules.
This process produces three distinct products: a liquid known as bio-oil or pyrolysis oil, a solid carbon-rich residue called biochar, and a gaseous mixture similar to syngas. Because it's an endothermic process (requiring energy input), the resulting products retain a very high energy content.
Understanding the Trade-offs
Each process comes with a distinct set of operational realities and strategic benefits. Choosing the right one depends on balancing complexity, cost, and desired outputs.
Simplicity vs. Versatility
Incineration is the most mature and technologically simplest of the three, making it a robust solution for waste destruction and heat generation.
Gasification and pyrolysis are more complex to operate. They require more precise control over temperature and feedstock quality but offer the significant advantage of producing more versatile outputs like fuels and chemical feedstocks.
Energy Output: Direct Heat vs. Stored Fuel
The energy from incineration is released immediately as heat. This is highly efficient if there is a direct use for that heat or steam nearby, such as in a power plant or district heating system.
Gasification and pyrolysis create intermediate fuels (syngas, bio-oil). These fuels can be stored, transported, and used more flexibly, but converting them into final energy (like electricity) involves additional steps and potential efficiency losses.
Final Products: Ash vs. Value-Added Materials
Incineration's primary solid output is ash, which typically must be landfilled.
Pyrolysis, in contrast, produces biochar, a valuable product that can be used as a soil amendment to improve fertility and sequester carbon. This transforms a waste stream into a valuable resource.
Making the Right Choice for Your Goal
Selecting the appropriate technology hinges entirely on your primary objective, whether it's waste management, energy production, or material recovery.
- If your primary focus is maximum waste volume reduction and direct heat generation: Incineration is the most direct and established pathway.
- If your primary focus is producing a versatile fuel gas for electricity or chemical synthesis: Gasification is the targeted technology for converting solid feedstocks into syngas.
- If your primary focus is creating liquid fuels, chemicals, or valuable solid co-products like biochar: Pyrolysis offers the unique ability to transform organic material into distinct liquid and solid outputs.
Ultimately, your decision depends on whether you view the input material as a problem to be eliminated or a resource to be transformed.
Summary Table:
| Technology | Oxygen Level | Primary Goal | Main Products |
|---|---|---|---|
| Incineration | Excess Oxygen | Waste Destruction & Heat | Heat, CO₂, Ash |
| Gasification | Limited Oxygen | Fuel Gas Production | Syngas (CO, H₂) |
| Pyrolysis | No Oxygen | Material Transformation | Bio-oil, Biochar, Syngas |
Ready to choose the right thermal conversion technology for your lab or project?
At KINTEK, we specialize in advanced laboratory equipment for researching and developing processes like pyrolysis and gasification. Whether you're exploring biochar production, syngas analysis, or new material synthesis, our precise and reliable systems are designed to meet your exact needs.
Let our experts help you unlock the potential of your materials. Contact KINTEK today to discuss your specific application and discover the perfect solution for your laboratory.
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- What are the risks of pyrolysis? Key Challenges in Waste-to-Energy Conversion
- What is the disposal of solid waste by pyrolysis? A Waste-to-Wealth Transformation Guide
- What is the limitations of pyrolysis? Key Economic and Technical Challenges to Consider
- What are the waste yields of pyrolysis? Turn Waste into Value with High-Efficiency Conversion
- What is the pyrolysis method for plastic waste? Convert Non-Recyclable Plastics into Fuel
- What is the history of pyrolysis technology? From Wood Distillation to Modern Waste Valorization
- What are the different types of pyrolysis reactors? Choose the Right Reactor for Your Process
- What is the difference between calcining and roasting? A Guide to High-Temperature Processing



















