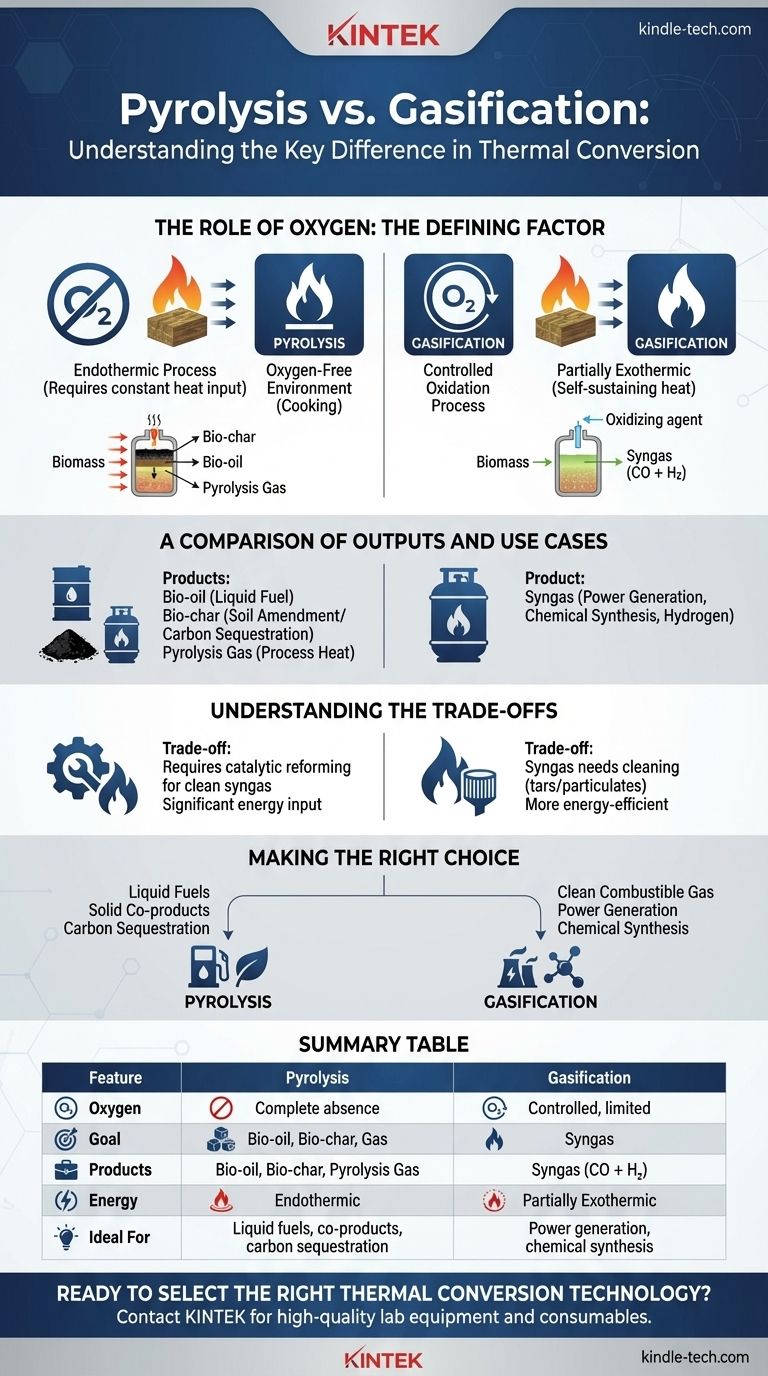
In the world of thermal conversion, the fundamental difference between pyrolysis and gasification lies in a single, critical element: oxygen. Pyrolysis is the thermal decomposition of material in the complete absence of oxygen, while gasification uses a controlled, limited amount of oxygen to partially oxidize the material. This core difference dictates the entire process, from the energy required to the final products generated.
The presence or absence of oxygen is not just a technical detail; it's the defining factor that determines the output. Pyrolysis is designed to break down materials into a portfolio of products (bio-oil, bio-char, and gas), while gasification is optimized to convert nearly all the material into a single gaseous fuel: syngas.

The Role of Oxygen: The Defining Factor
The core chemistry of each process is determined by how it uses—or avoids—oxygen. This distinction is the source of all other differences.
Pyrolysis: An Oxygen-Free Environment
Pyrolysis is essentially "cooking" feedstock like biomass in a sealed, oxygen-free vessel. Because there is no oxygen, the material does not combust.
Instead, the intense heat breaks down the complex organic polymers into a mix of smaller, valuable components. This is primarily an endothermic process, meaning it requires a constant input of energy to sustain the reaction.
Gasification: A Controlled Oxidation Process
Gasification introduces a very specific, limited amount of an oxidizing agent (usually oxygen, air, or steam) into the reactor. This is not enough oxygen for full combustion, which would simply burn the fuel and release heat and CO2.
Instead, it allows for partial oxidation. This generates enough heat to make the process self-sustaining (partially exothermic) while converting the feedstock into synthesis gas, or syngas.
A Comparison of Outputs and Use Cases
The different chemical environments of pyrolysis and gasification result in fundamentally different product streams, each suited for different applications.
The Products of Pyrolysis: A Diverse Portfolio
Pyrolysis breaks feedstock down into three primary products:
- Bio-char: A solid, carbon-rich charcoal-like substance used for soil amendment and carbon sequestration.
- Bio-oil (Pyrolysis Oil): A liquid mixture of hydrocarbons that can be upgraded into transportation fuels or used to produce chemicals.
- Pyrolysis Gas: A mix of flammable gases (hydrogen, methane, carbon monoxide) that can be used to generate the heat needed to power the pyrolysis process itself.
This process is ideal when you want to create a range of valuable solid and liquid co-products, not just a single gas.
The Product of Gasification: A Singular Focus on Syngas
The primary goal of gasification is to maximize the conversion of feedstock into a single product: syngas.
Syngas is a mixture composed mainly of carbon monoxide (CO) and hydrogen (H2). It is a highly versatile fuel and chemical building block that can be used to:
- Generate electricity in gas turbines or engines.
- Be catalytically converted into hydrogen, ethanol, or synthetic diesel.
- Serve as a feedstock for the chemical industry.
This process is the clear choice when your sole objective is to produce a large volume of combustible gas for power or synthesis.
Understanding the Trade-offs
Neither process is inherently superior; they are simply engineered for different outcomes. Choosing between them involves understanding their operational trade-offs.
Product Purity and Post-Processing
The gas produced from pyrolysis contains complex hydrocarbons and other compounds. To create a clean syngas from it, an additional step like catalytic reforming is often required.
Gasification is designed to produce syngas more directly, but this gas still contains tars and particulates that must be cleaned before it can be used in sensitive equipment like engines or fuel cells.
Energy Balance
As a primarily endothermic process, pyrolysis requires a significant and continuous external heat source to run.
Gasification's partial oxidation reactions generate their own heat, which can make the overall process more energy-efficient and self-sustaining once it reaches operating temperature.
Making the Right Choice for Your Goal
Selecting the correct technology depends entirely on your desired end product.
- If your primary focus is producing liquid fuels or valuable solid co-products: Pyrolysis is the superior choice, as it is designed to yield bio-oil and bio-char alongside a fuel gas.
- If your primary focus is generating a clean, combustible gas for power or chemical synthesis: Gasification is the more direct and efficient pathway, as its entire purpose is to maximize the conversion of feedstock into syngas.
- If your primary focus is maximizing carbon sequestration: Pyrolysis offers a unique advantage by producing stable bio-char, which can lock carbon away in the soil for centuries.
Understanding this fundamental difference in chemistry and intent is the key to selecting the right thermal conversion technology for your specific objective.
Summary Table:
| Feature | Pyrolysis | Gasification |
|---|---|---|
| Oxygen Environment | Complete absence of oxygen | Controlled, limited oxygen |
| Primary Goal | Produce multiple products (bio-oil, bio-char, gas) | Produce a single product (syngas) |
| Primary Products | Bio-oil, Bio-char, Pyrolysis Gas | Syngas (CO + H₂) |
| Process Energy | Endothermic (requires external heat) | Partially Exothermic (self-sustaining) |
| Ideal For | Liquid fuels, solid co-products, carbon sequestration | Power generation, chemical synthesis |
Ready to select the right thermal conversion technology for your lab or project?
The choice between pyrolysis and gasification is critical for achieving your specific goals, whether it's producing valuable bio-oils or generating clean syngas for power. At KINTEK, we specialize in providing high-quality lab equipment and consumables to support your research and development in these advanced processes.
Our experts can help you navigate the complexities of thermal conversion technology. Contact us today using the form below to discuss how our solutions can enhance your laboratory's efficiency and success.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the temperature for activated carbon regeneration? Key Ranges from 220°C to 900°C
- What temperature is needed for porcelain? A Guide to Cone 6 and Cone 10 Firing
- Can you restore activated carbon? Understanding the Industrial Reactivation Process
- What are the principles of a rotary kiln? Master the Mechanics of High-Temperature Processing
- What is the temperature of a carbon regeneration kiln? Mastering the 750-800°C Reactivation Process



















