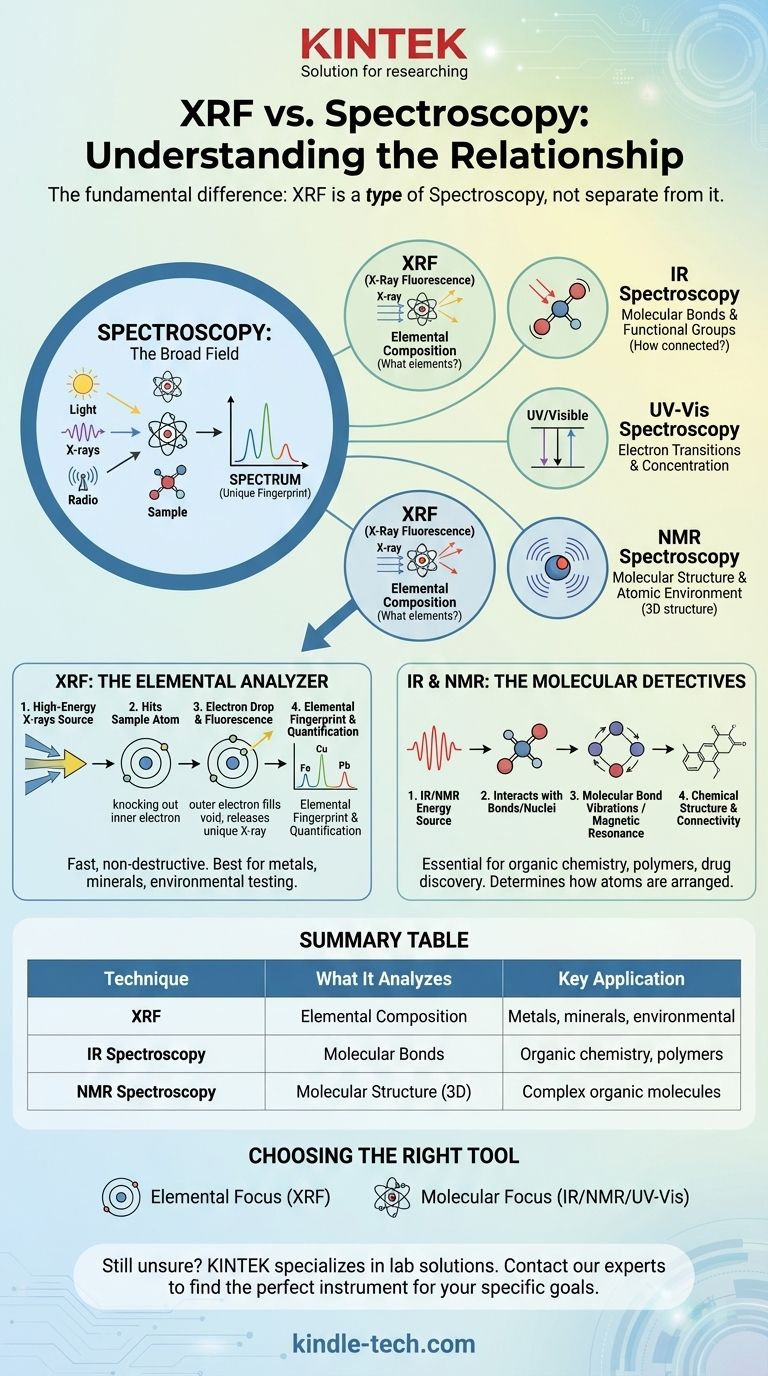
The fundamental difference is that X-Ray Fluorescence (XRF) is not separate from spectroscopy; it is a specific type of spectroscopy. Spectroscopy is the broad field of studying how energy and matter interact, while XRF is a single, powerful technique within that field used for determining the elemental composition of a material.
The core misunderstanding is to view these as opposing choices. The correct mental model is to see "spectroscopy" as the overarching discipline and "XRF" as one of many specialized tools within that discipline, each defined by the type of energy it uses and the information it reveals.

What is Spectroscopy? The Fundamental Principle
The Study of Interaction
Spectroscopy, at its heart, is the study of the interaction between some form of energy (like light, X-rays, or radio waves) and matter.
When energy hits a sample, the sample absorbs some of it and emits the rest. By measuring what is emitted or absorbed, we can learn a great deal about the sample's properties.
The Spectrum: A Unique Fingerprint
The result of a spectroscopic measurement is a spectrum, which is typically a graph plotting energy intensity versus wavelength or energy level.
This spectrum acts like a unique fingerprint. Different atoms and molecules will interact with energy in their own distinct way, producing a characteristic pattern that allows us to identify them.
Where XRF Fits In: A Specific Spectroscopic Technique
The Energy Source: High-Energy X-rays
XRF is a form of emission spectroscopy that uses high-energy X-rays as its energy source. An XRF instrument directs a primary beam of X-rays at the surface of a sample.
The Sample Interaction: Atomic Fluorescence
This incoming energy is strong enough to knock an electron out of one of the atom's inner electron shells. This creates an unstable vacancy.
To regain stability, an electron from a higher-energy outer shell immediately drops down to fill the hole. As it drops, it releases its excess energy in the form of a secondary, or "fluorescent," X-ray.
The Result: An Elemental Fingerprint
Critically, the energy of this fluorescent X-ray is unique to the element from which it was emitted. The XRF detector measures the energies of all the secondary X-rays coming from the sample.
By analyzing this spectrum of fluorescent X-rays, the instrument can identify precisely which elements are present and in what quantity.
A Broader Look: Other Types of Spectroscopy
To clarify XRF's role, it helps to compare it to other common spectroscopic techniques that answer different questions.
Infrared (IR) Spectroscopy
IR spectroscopy uses lower-energy infrared light to probe the vibrations of chemical bonds within a molecule. It is excellent for identifying functional groups and determining a molecule's structure.
UV-Visible (UV-Vis) Spectroscopy
This technique uses ultraviolet and visible light to study electron transitions between orbitals in molecules. It is often used to determine the concentration of a substance in a solution.
Nuclear Magnetic Resonance (NMR) Spectroscopy
NMR uses radio waves within a powerful magnetic field to probe the chemical environment of atomic nuclei (like hydrogen or carbon). It is one of the most powerful tools for determining the precise 3D structure of complex organic molecules.
Understanding the Trade-offs: The Question Dictates the Tool
The key difference is the question each technique is designed to answer. Choosing the "right" one depends entirely on your goal.
XRF: The Elemental Analyzer
XRF tells you what elements are in a sample and how much of each is present. It is fast, non-destructive, and exceptionally powerful for analyzing metals, minerals, soil, and consumer products.
However, XRF generally cannot tell you how those elements are bonded together. It can identify iron (Fe), but it cannot distinguish between different iron oxides like rust (Fe₂O₃) and magnetite (Fe₃O₄).
IR & NMR: The Molecular Detectives
Techniques like IR and NMR tell you how atoms are connected to form molecules. They are the essential tools of organic chemistry, polymer science, and drug discovery.
They can distinguish between graphite and diamond (both pure carbon) because they can detect the different chemical bonding arrangements. However, they are not typically used for simple elemental analysis of a metal alloy.
Making the Right Choice for Your Goal
- If your primary focus is elemental composition: Use XRF. It is the direct and efficient choice for metallurgy, geology, environmental testing, and regulatory compliance (e.g., checking for lead in toys).
- If your primary focus is molecular structure and identification: Use IR or NMR spectroscopy. These are the necessary tools for chemical synthesis, pharmaceutical analysis, and materials science.
- If your primary focus is a complete characterization: You often need multiple methods. A comprehensive analysis might use XRF to find the elemental building blocks and then IR to understand how they are assembled.
Ultimately, understanding this distinction empowers you to move from choosing a tool to asking the right analytical question.
Summary Table:
| Technique | What It Analyzes | Key Application |
|---|---|---|
| XRF (X-Ray Fluorescence) | Elemental Composition (what elements are present) | Metals, minerals, environmental testing, consumer goods |
| IR Spectroscopy | Molecular Bonds & Functional Groups (how atoms are connected) | Organic chemistry, polymer science, drug discovery |
| NMR Spectroscopy | Molecular Structure & Atomic Environment (3D structure) | Determining complex organic molecule structures |
Still unsure which analytical technique is right for your application?
KINTEK specializes in lab equipment and consumables, serving the precise needs of laboratories like yours. Our experts can help you select the perfect instrument—whether it's an XRF analyzer for elemental quantification or another spectroscopic tool—to ensure accurate and efficient results for your specific materials and goals.
Contact our specialists today for a personalized consultation and discover the right solution for your lab's challenges.
Visual Guide
Related Products
- Customizable XRD Sample Holders for Diverse Research Applications
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Three-dimensional electromagnetic sieving instrument
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Optical Window Glass Substrate Wafer CaF2 Substrate Window Lens
People Also Ask
- What is the effect of sintering on hardness? Maximize Material Strength & Durability
- What are the precautions to be taken during blending of metal powders? Ensure Safety and Quality in Your Lab
- What is the effect of cooling rate on casting? Control Strength, Ductility, and Defects
- How thick is gold sputter coating? Optimize Your SEM Sample Preparation for Clear Images
- What is the role of a blast drying oven in COF synthesis? Driving High-Crystallinity Solvothermal Reactions
- What is the difference between conventional and microwave pyrolysis? Unlock Faster, More Efficient Heating
- What role does a precision laboratory drying oven play in the synthesis of GO-PANI nanocomposites? Protect Material Integrity
- What are the heat treatment processes in the heat treatment of steel? Master the Methods for Superior Material Properties















