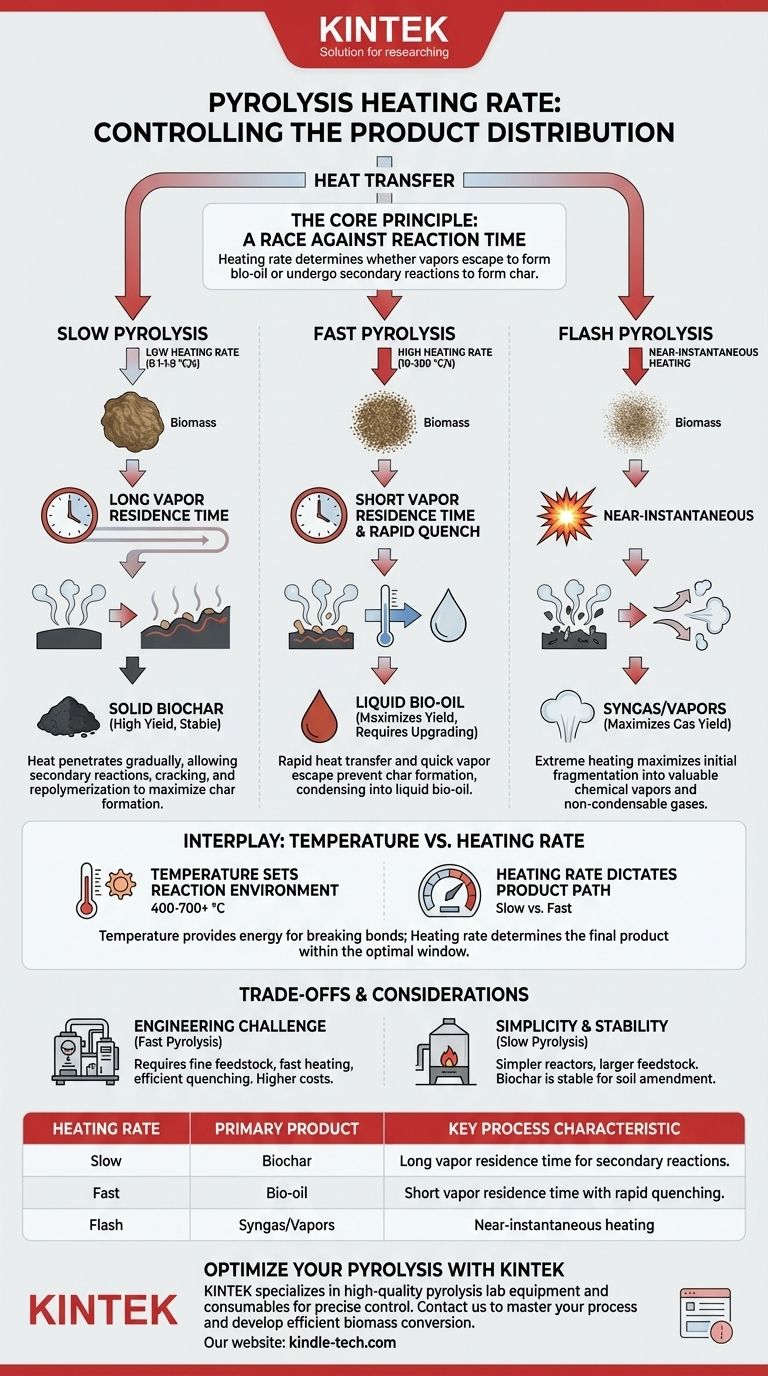
In pyrolysis, the heating rate is the primary control variable used to determine the final product distribution. Slower heating rates fundamentally favor the production of solid biochar, while rapid heating rates are essential for maximizing the yield of liquid bio-oil. This effect is a direct result of how heating speed influences the reaction pathways and the time available for secondary reactions to occur.
The core principle is a race against time. The heating rate determines whether volatile vapors produced during initial decomposition have time to undergo secondary reactions that form more char and gas, or if they escape the hot zone quickly and are condensed into liquid bio-oil.

The Core Principle: A Race Against Reaction Time
Pyrolysis involves the thermal decomposition of biomass in the absence of oxygen. The process can be steered toward different products—solid, liquid, or gas—by controlling the operating parameters, with heating rate being one of the most critical.
Slow Pyrolysis: Maximizing Biochar
In slow pyrolysis, the heating rate is very low (e.g., 0.1-1.0 °C/s). This slow addition of energy allows heat to penetrate deep into the biomass particles gradually.
This process gives ample time for secondary reactions to occur. As volatile vapors are released, they linger in the hot reaction zone, interacting with the hot surface of the nascent char. These interactions lead to further cracking and repolymerization, ultimately depositing more carbon and forming a high-yield, stable biochar.
Fast Pyrolysis: Maximizing Bio-oil
Fast pyrolysis uses extremely high heating rates (e.g., 10-200 °C/s or higher). The goal is to transfer heat to the surface of the biomass particle as quickly as possible.
This rapid heating creates a very short vapor residence time. The volatile compounds are vaporized and escape the particle and the hot reactor zone before they have a chance to undergo those secondary char-forming reactions. These vapors are then rapidly cooled, or "quenched," to condense them into a liquid known as bio-oil (or pyrolysis oil).
Flash Pyrolysis: Pushing Toward Vapors and Gases
Flash pyrolysis represents the extreme end of the spectrum, with near-instantaneous heating rates. This process is designed to maximize the initial fragmentation of the biomass into valuable chemical vapors and non-condensable gases (syngas), often minimizing both liquid and solid fractions.
The Interplay Between Heating Rate and Temperature
While often discussed together, it is crucial to distinguish between the final pyrolysis temperature and the heating rate. They are independent variables that have distinct but related effects.
Temperature Sets the Reaction Environment
The final process temperature dictates the overall energy available for breaking chemical bonds. As noted, higher temperatures (e.g., >700 °C) provide enough energy to crack all products, favoring the production of syngas. Lower temperatures (e.g., 400-550 °C) are the typical target for producing either biochar or bio-oil.
Heating Rate Dictates the Product Path
The heating rate determines which product you get within that optimal temperature window. You can run a process at a final temperature of 500 °C, but if you get there slowly, you will produce mostly biochar. If you reach 500 °C very quickly, you will produce mostly bio-oil.
Understanding the Trade-offs
Choosing a heating rate is not just a scientific decision; it is an engineering one with significant practical trade-offs.
The Engineering Challenge of Fast Pyrolysis
Maximizing bio-oil yield requires sophisticated engineering. Reactors must handle very fine feedstock (to ensure rapid heat transfer), achieve incredibly fast heating, and include an efficient system for quenching vapors. This often leads to higher capital and operational costs.
The Simplicity of Slow Pyrolysis
Processes designed for biochar are generally simpler and more robust. They can accommodate larger feedstock particles and use simpler reactor designs like kilns and retorts. This makes the technology more accessible and often more reliable for decentralized applications.
Product Quality and Stability
Fast pyrolysis bio-oil is acidic, corrosive, and chemically unstable, typically requiring immediate upgrading to be used as a fuel. In contrast, the biochar from slow pyrolysis is a highly stable carbon product that can be used directly as a soil amendment or solid fuel.
Choosing the Right Process for Your Goal
Your choice of heating rate must be directly aligned with your desired end product.
- If your primary focus is soil amendment or solid fuel (biochar): You must use a slow heating rate to maximize solid yield and carbon stability.
- If your primary focus is producing liquid biofuels or chemical feedstocks (bio-oil): You must use a fast heating rate and rapid quenching to maximize liquid yield.
- If your primary focus is generating syngas for heat or power: You should use very high temperatures combined with a fast heating rate to maximize the cracking of all organic matter into gas.
Ultimately, mastering the heating rate is the key to unlocking the specific value you wish to extract from biomass.
Summary Table:
| Heating Rate | Primary Product | Key Process Characteristic |
|---|---|---|
| Slow (0.1-1.0 °C/s) | Biochar | Long vapor residence time for secondary char-forming reactions. |
| Fast (10-200 °C/s) | Bio-oil | Short vapor residence time with rapid quenching to condense liquids. |
| Flash (Very High) | Syngas/Vapors | Near-instantaneous heating to maximize gas yield. |
Ready to optimize your pyrolysis process for maximum yield?
The choice of heating rate is critical to achieving your target product, whether it's stable biochar for soil amendment, liquid bio-oil for fuel, or syngas for energy. The right lab equipment is essential for precise control and repeatable results.
KINTEK specializes in high-quality pyrolysis lab equipment and consumables, helping researchers and engineers like you accurately control heating rates and other critical parameters. We provide the reliable tools you need to develop and scale your biomass conversion processes efficiently.
Contact us today to discuss your specific application and how our solutions can help you master your pyrolysis process. Get in touch via our contact form to speak with an expert.
Visual Guide
Related Products
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the industrial application of calcination? Transforming Raw Materials for Manufacturing
- What can pyrolysis be used for? Transform Waste into Fuel, Biochar, and Syngas
- What are the advantages of using a rotary tube furnace for MoVOx catalysts? Elevate Uniformity and Crystallinity
- What is the difference between fast and slow biomass pyrolysis? Optimize Your Biofuel or Biochar Production
- What is biomass and explain the process of biomass pyrolysis? A Guide to Converting Waste into Valuable Resources
- How is pyrolysis sustainable? Turning Waste into Energy and Circular Materials
- What are the applications of calcination? A Guide to Thermal Processing in Industry
- How efficient is fast pyrolysis? Maximizing Biomass Conversion with High-Yield Bio-Oil Production



















