While an exceptionally powerful technique for elemental analysis, X-ray Fluorescence (XRF) is not without its constraints. Its primary limitations involve an inability to effectively detect very light elements, challenges in measuring trace-level concentrations, and its nature as a surface-only analysis, which can be misleading for non-homogenous materials.
The core challenge with XRF is not that it has flaws, but that its physical principles create a specific set of operational boundaries. Understanding these boundaries is the key to using the technique effectively and avoiding misinterpretation of your data.

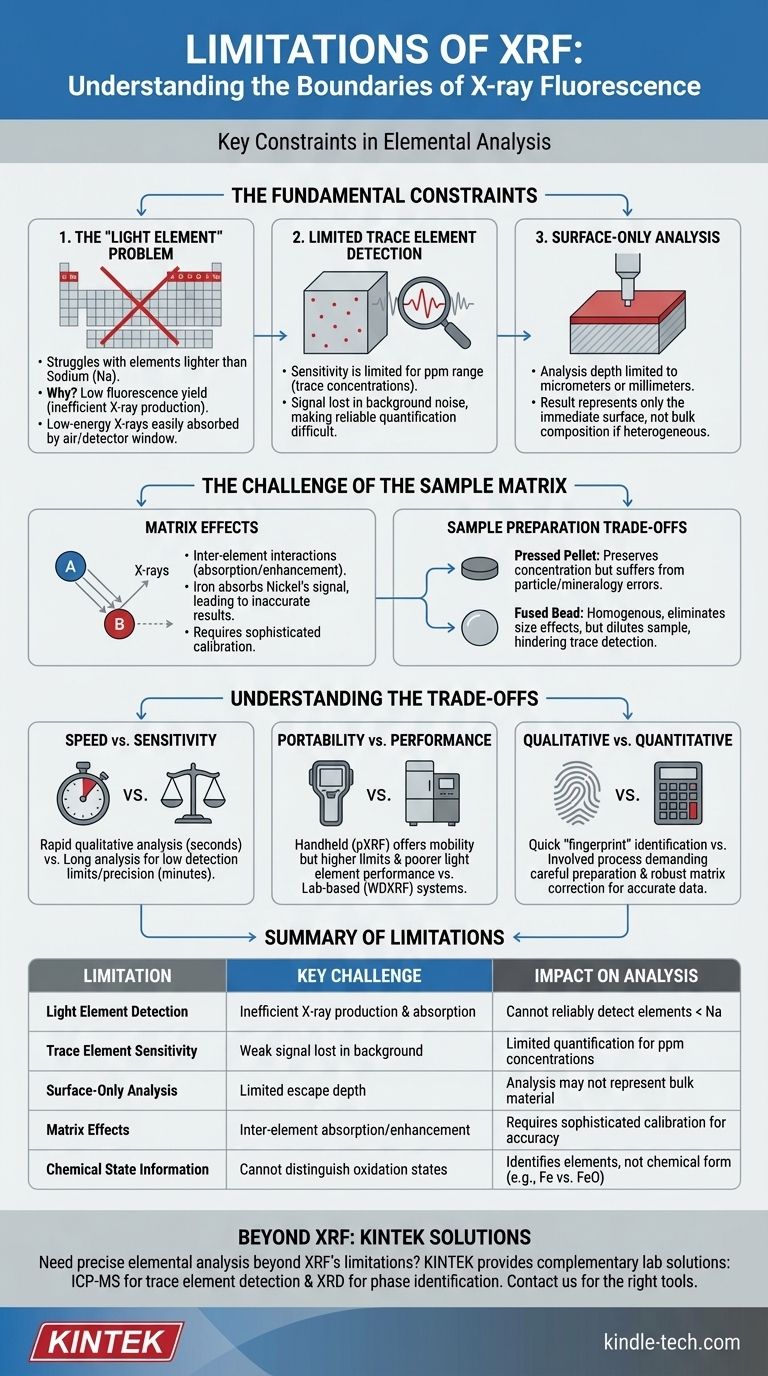
The Fundamental Constraints of XRF
The most significant limitations are not related to specific instrument models but are rooted in the physics of how X-rays interact with matter.
The "Light Element" Problem
XRF struggles to detect elements lighter than sodium (Na) on the periodic table. This happens for two main physical reasons.
First, very light elements have a low "fluorescence yield," meaning they are inefficient at producing characteristic X-rays when excited.
Second, the X-rays they do produce are very low-energy (long-wavelength) and are easily absorbed by the air path between the sample and the detector, or even by the detector window itself.
Limited Detection of Trace Elements
While excellent for major and minor elements, XRF's sensitivity for trace elements (typically in the parts-per-million range) can be limited.
The signal from a low-concentration element can be weak and easily lost in the background noise generated by X-ray scattering from the sample itself. This makes it difficult to reliably quantify elements that are present in very small amounts.
Surface-Only Analysis
XRF is fundamentally a surface-sensitive technique. The depth from which fluorescent X-rays can escape and reach the detector is typically limited to micrometers or, at most, a few millimeters, depending on the sample's density and composition.
This means the analysis only represents the composition of the sample's immediate surface. If the bulk material is different from the surface (due to contamination, corrosion, or natural heterogeneity), the XRF result will not be representative of the whole.
The Challenge of the Sample Matrix
Beyond fundamental physics, the composition and preparation of the sample itself—known as the "matrix"—introduce another layer of limitations.
Matrix Effects: The Hidden Variable
The accuracy of XRF quantification is heavily impacted by matrix effects. These are inter-element interactions where X-rays emitted by one element are either absorbed or enhanced by another element within the sample.
For example, iron in a sample will strongly absorb the fluorescent X-rays from nickel, causing the instrument to report a lower nickel concentration than is actually present. Correcting for these effects requires sophisticated software and well-matched calibration standards.
The Impact of Sample Preparation
How you prepare your sample creates a critical trade-off. For powdered samples, two common methods are pressed pellets and fused beads.
Using a pressed pellet preserves the original concentration but can suffer from errors due to particle size differences and mineralogy.
Creating a fused bead involves melting the sample with a flux to create a perfectly homogenous glass disk. This eliminates particle size effects but significantly dilutes the sample. As a result, this method makes it much more difficult, or even impossible, to detect trace elements.
Inability to Distinguish Chemical States
A standard XRF analysis identifies which elements are present and in what quantity, but it provides no information about their chemical form or oxidation state.
For instance, XRF can tell you the total concentration of iron (Fe), but it cannot distinguish between metallic iron, iron(II) oxide (FeO), or iron(III) oxide (Fe₂O₃).
Understanding the Trade-offs
Choosing to use XRF means accepting a set of practical trade-offs.
Speed vs. Sensitivity
XRF is known for its incredible speed, often providing a qualitative analysis in seconds. However, achieving the lowest possible detection limits and highest precision requires much longer analysis times, sometimes many minutes per sample.
Portability vs. Performance
Handheld XRF (pXRF) analyzers offer immense value for field screening but come with compromises. They typically have higher detection limits, poorer performance for light elements, and are more susceptible to errors from irregular sample surfaces compared to high-power, lab-based wavelength-dispersive (WDXRF) systems.
Qualitative vs. Quantitative Analysis
Getting a quick, qualitative "fingerprint" of a sample's elemental makeup is a primary strength of XRF. Obtaining truly accurate, reliable quantitative data, however, is a much more involved process that demands careful sample preparation and robust matrix correction.
Making the Right Choice for Your Application
Use these limitations to guide your decision on whether XRF is the correct tool for your analytical goal.
- If your primary focus is rapid screening and major element identification: XRF is an exceptional tool, but be wary of trusting quantitative numbers without proper calibration for your specific matrix.
- If your primary focus is quantifying trace elements (ppm-level): You may need to use a different technique with higher sensitivity, such as Inductively Coupled Plasma (ICP-MS or ICP-OES).
- If your primary focus is analyzing very light elements (e.g., lithium, beryllium, boron): XRF is not the appropriate technique, and other methods are required.
- If your primary focus is understanding chemical structure or mineral phases: You must supplement XRF with a complementary technique like X-ray Diffraction (XRD) or Raman spectroscopy.
By respecting its boundaries, you can leverage the distinct power and speed of XRF to its full potential.
Summary Table:
| Limitation | Key Challenge | Impact on Analysis |
|---|---|---|
| Light Element Detection | Inefficient X-ray production & absorption by air | Cannot reliably detect elements lighter than sodium (Na) |
| Trace Element Sensitivity | Weak signal lost in background noise | Limited quantification for parts-per-million (ppm) concentrations |
| Surface-Only Analysis | Limited escape depth of fluorescent X-rays | Analysis may not represent bulk material if surface is heterogeneous |
| Matrix Effects | Inter-element absorption/enhancement of X-rays | Requires sophisticated calibration for accurate quantification |
| Chemical State Information | Cannot distinguish oxidation states | Identifies elements but not their chemical form (e.g., Fe vs. FeO) |
Need precise elemental analysis beyond XRF's limitations? KINTEK specializes in lab equipment and consumables, serving laboratory needs with solutions that complement XRF, such as ICP-MS for trace element detection or XRD for phase identification. Let our experts help you select the right tools for accurate, reliable results. Contact us today to discuss your specific application and enhance your analytical capabilities!
Visual Guide
Related Products
- Customizable XRD Sample Holders for Diverse Research Applications
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Three-dimensional electromagnetic sieving instrument
- Lab Internal Rubber Mixer Rubber Kneader Machine for Mixing and Kneading
People Also Ask
- What is the difference between XRF and XRD techniques? A Guide to Choosing the Right Analytical Tool
- How should a sample holder be handled to ensure its longevity? Protect Your Lab Investment and Data Integrity
- What are the limitations of the IR spectroscopy? Understanding Its Boundaries for Accurate Analysis
- Why is an airtight sample holder with a beryllium window required for XRD of sulfide solid electrolytes?
- What are the factors that affect melting and boiling point? Unlock the Science of Phase Transitions















