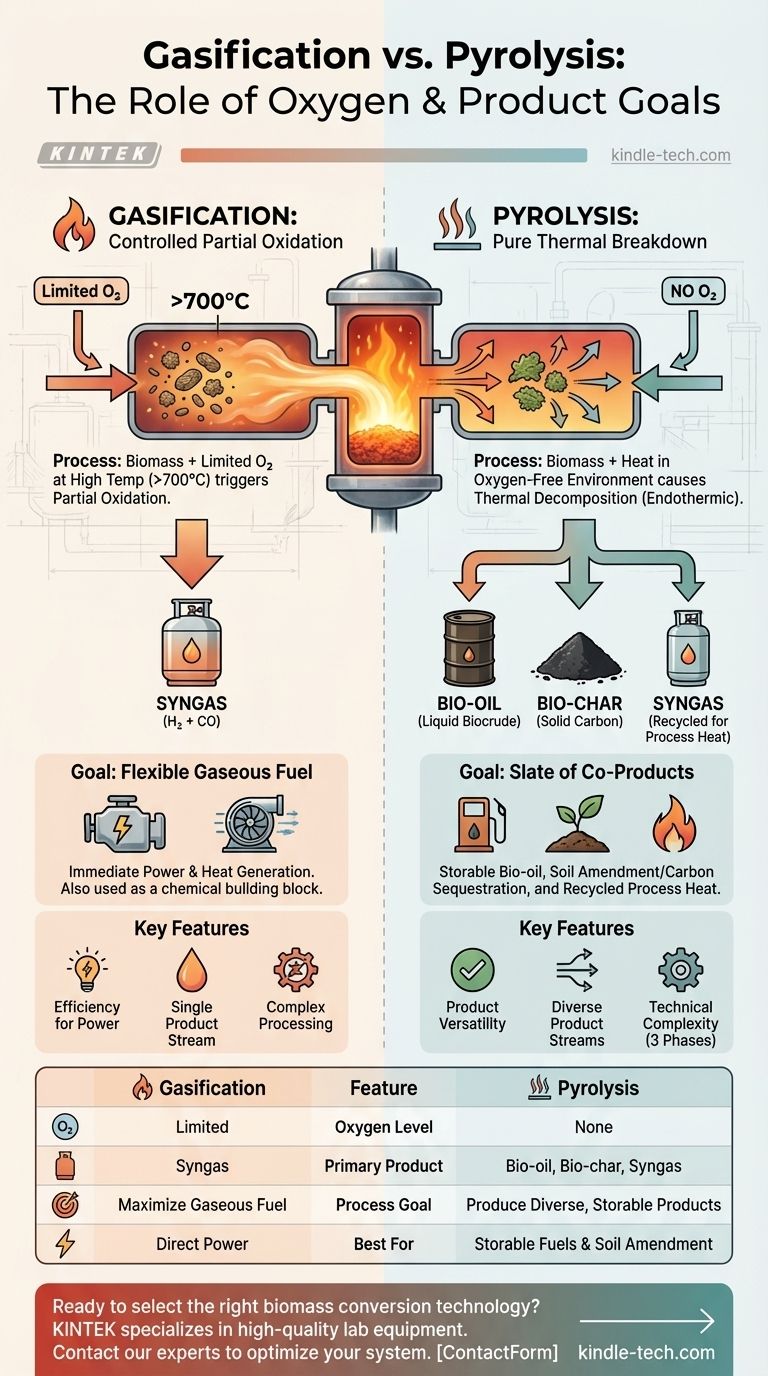
At a fundamental level, the main difference between gasification and pyrolysis is the presence of oxygen. Gasification uses a limited, controlled amount of oxygen at high temperatures to convert biomass primarily into a combustible gas. Pyrolysis, in contrast, is the thermal decomposition of biomass in the complete absence of oxygen, which breaks it down into a mix of liquid bio-oil, solid bio-char, and gas.
This distinction is not just a technical detail; it defines the core purpose of each process. Gasification is engineered to maximize the output of a single product—gaseous fuel (syngas)—for immediate power and heat generation. Pyrolysis is engineered to create a diverse slate of valuable and storable products: liquid, solid, and gas.

The Role of Oxygen: Oxidation vs. Thermal Decomposition
The decision to include or exclude oxygen fundamentally changes the chemical reactions that take place, the energy balance of the system, and the nature of the final products.
Gasification: Controlled Partial Oxidation
In gasification, a feedstock like biomass is subjected to very high temperatures (typically above 700°C) with an insufficient amount of oxygen for full combustion.
This "starved oxygen" environment triggers partial oxidation. This process releases some energy, helping to drive the reaction, while breaking down the biomass into a primary product: synthesis gas, or syngas. Syngas is a mixture of mainly hydrogen (H₂) and carbon monoxide (CO).
Pyrolysis: Pure Thermal Breakdown
Pyrolysis is a strictly thermochemical process. By heating biomass in an oxygen-free (anaerobic) environment, you prevent combustion from occurring.
Instead of burning, the heat breaks the complex organic polymers of the biomass into smaller molecules. Because there is no oxidation, this process is primarily endothermic, meaning it requires a consistent external energy source to continue. The resulting products retain a high portion of the original energy content of the feedstock.
A Tale of Two Product Streams
The different chemistries of gasification and pyrolysis lead to entirely different sets of outputs, each suited for distinct applications.
The Goal of Gasification: A Flexible Gaseous Fuel
The primary output of gasification is syngas. This is an incredibly flexible intermediate product.
It can be combusted directly in gas engines or turbines to generate electricity and heat efficiently. It can also be used as a chemical building block to synthesize liquid fuels (like diesel) or valuable chemicals (like methanol and ammonia).
The Goal of Pyrolysis: A Slate of Co-Products
Pyrolysis is not designed to maximize one output, but to create three distinct and valuable product streams simultaneously.
- Bio-oil: A liquid, sometimes called pyrolysis oil or biocrude, that can be stored, transported, and upgraded into renewable transportation fuels like gasoline and diesel.
- Bio-char: A stable, carbon-rich solid that is an excellent soil amendment, improving water retention and nutrient availability. It also serves as a powerful method for long-term carbon sequestration.
- Syngas: A smaller volume of non-condensable gases, which is often recycled to provide the process heat required to run the pyrolysis reactor, making the system more self-sufficient.
Understanding the Trade-offs
Choosing between these technologies involves weighing the efficiency of energy conversion against the versatility of the products.
Efficiency for Power Generation
For the direct production of electricity and heat, gasification is generally considered the more efficient route. The process is optimized to convert the maximum amount of the feedstock's energy into a single, combustible gas ready for use in power generation systems.
Versatility of Products
Pyrolysis offers superior product versatility. Instead of committing all of the biomass energy to immediate power generation, you create a portfolio of products. Storable bio-oil acts like a renewable crude, and bio-char has its own distinct economic and environmental value. This flexibility can be a significant advantage, depending on market conditions.
Process Complexity
The output of gasification is a single stream of gas, which can simplify downstream processing. Pyrolysis, however, produces outputs in three different phases (solid, liquid, and gas) that must be collected, separated, and processed, which can add technical complexity to the overall system.
Making the Right Choice for Your Goal
Your choice between gasification and pyrolysis should be driven by your end objective, not by the processes themselves.
- If your primary focus is immediate electricity or process heat: Gasification is the more direct and efficient pathway, converting biomass into a combustible syngas designed for power systems.
- If your primary focus is producing storable liquid fuels or valuable co-products like bio-char: Pyrolysis is the superior choice, as it is specifically designed to break biomass down into bio-oil and bio-char.
Understanding this core difference in process and purpose is the key to selecting the right technology for your specific energy or material goal.
Summary Table:
| Feature | Gasification | Pyrolysis |
|---|---|---|
| Oxygen Level | Limited, controlled amount | Complete absence |
| Primary Product | Syngas (H₂ + CO) | Bio-oil, Bio-char, and Syngas |
| Process Goal | Maximize gaseous fuel for immediate power/heat | Produce diverse, storable products |
| Best For | Direct electricity and heat generation | Storable liquid fuels and soil amendment (bio-char) |
Ready to select the right biomass conversion technology for your lab or pilot project? The choice between gasification and pyrolysis is critical for achieving your energy and material goals. KINTEK specializes in providing high-quality lab equipment and consumables for both processes. Our experts can help you design and equip your system for optimal performance. Contact our team today to discuss your specific needs and discover how KINTEK can support your innovation in renewable energy and materials.
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What is the temperature range for pyrolysis? Optimize for Biochar, Bio-oil, or Syngas
- What is the difference between pyrolysis combustion and gasification? A Guide to Thermal Conversion Technologies
- How do high-temperature reaction furnaces control in-situ MMCs? Master Material Precision and Structural Integrity
- How is a high-temperature calcination furnace utilized in BZY20 Sol-gel? Achieve Pure Cubic Perovskite Phases
- What temperature is needed for pyrolysis waste? A Guide to Optimizing Your Waste-to-Value Process



















