At its core, Chemical Vapor Deposition (CVD) is a mechanism for building a solid material from a gas. The process involves introducing one or more volatile precursor gases into a reaction chamber where they decompose on a heated substrate. This chemical reaction deposits a thin, high-performance solid film onto the substrate's surface, while gaseous byproducts are removed.
The critical insight is that CVD is not a simple coating or spraying process. It is a controlled chemical reaction that "grows" a solid film on a surface, allowing for the precise construction of materials with high purity and specific crystalline structures.
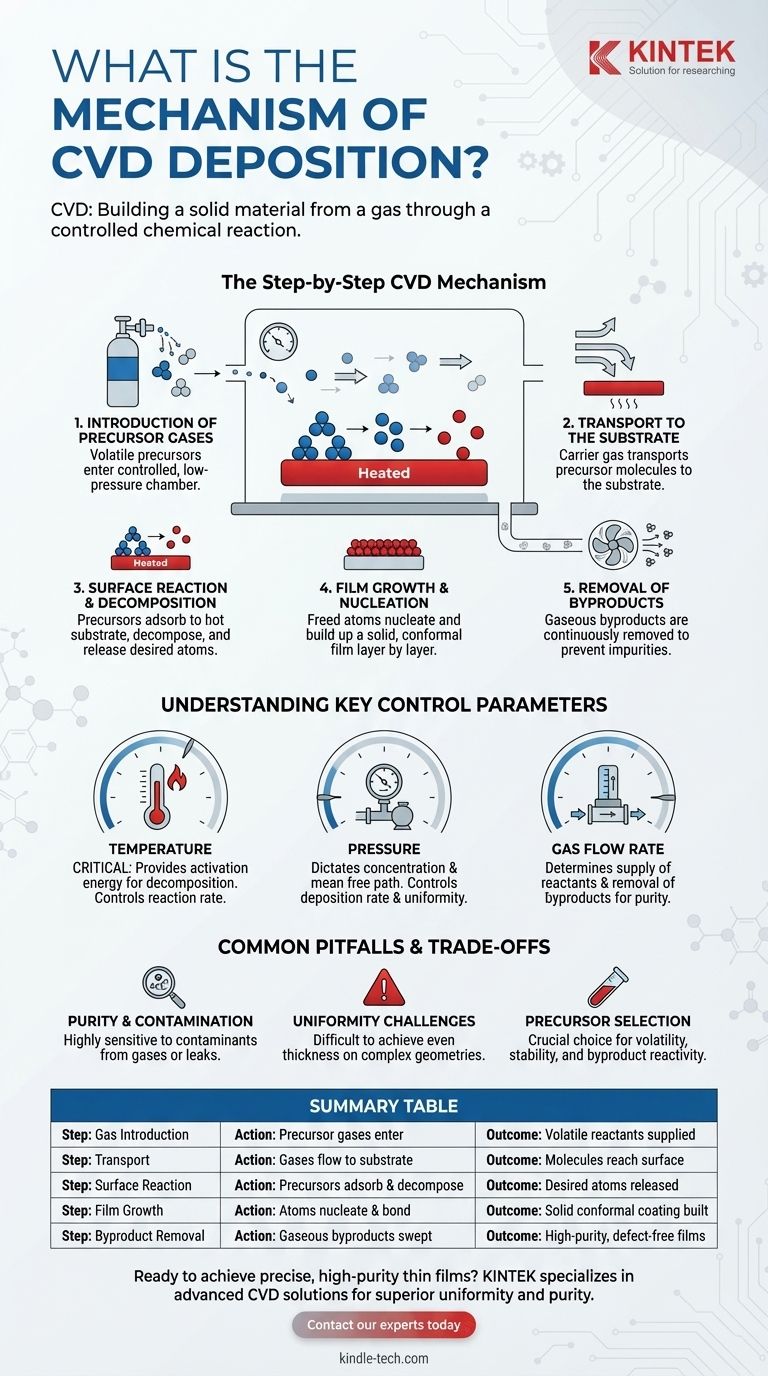
The Step-by-Step CVD Mechanism
To understand CVD, it's best to break it down into a sequence of distinct events. Each step is critical for controlling the quality and properties of the final material.
1. Introduction of Precursor Gases
The process begins by feeding specific precursor gases into a deposition chamber. These are volatile compounds that contain the chemical elements intended for the final film.
The chamber is typically held under a controlled, often low-pressure (vacuum), environment. This ensures process stability and minimizes contamination.
2. Transport to the Substrate
Once inside the chamber, a carrier gas or pressure differential transports the precursor molecules to the substrate. The substrate is the material or object onto which the film will be grown.
Gas flow rates are precisely managed to ensure a steady and uniform supply of reactants to the substrate surface.
3. Surface Reaction and Decomposition
This is the central event of the CVD mechanism. The substrate is heated to a specific reaction temperature, providing the thermal energy needed to initiate a chemical reaction.
When the precursor gases come into contact with the hot surface, they adsorb (temporarily stick) to it and decompose or react with other gases. This breaks the chemical bonds in the precursors, releasing the desired atoms.
4. Film Growth and Nucleation
The freed atoms bond to the substrate and to each other, forming a stable solid film. This process, known as nucleation, builds up layer by layer over time.
Because the reaction occurs directly on the surface, the film conforms precisely to the shape of the substrate, creating a uniform and dense coating.
5. Removal of Byproducts
The chemical reactions that form the solid film almost always create unwanted gaseous byproducts.
A continuous gas flow through the chamber is essential to sweep these byproducts away. This prevents them from getting incorporated into the growing film, which would create impurities and defects.
Understanding the Key Control Parameters
The quality of a CVD film is not accidental; it is a direct result of meticulously controlling the reaction environment. Understanding these parameters is key to understanding the process itself.
The Role of Temperature
Temperature is arguably the most critical variable. It provides the activation energy required for the precursor decomposition and surface reactions to occur. Too low, and the reaction won't happen; too high, and unwanted gas-phase reactions can occur, reducing film quality.
The Importance of Pressure
The pressure inside the chamber dictates the concentration of precursor molecules and their mean free path (the average distance a molecule travels before colliding with another). Controlling pressure is vital for managing the deposition rate and film uniformity.
The Function of Gas Flow Rate
Flow rates determine the supply rate of fresh precursor gases to the substrate and, just as importantly, the rate at which byproducts are removed. This balance is crucial for achieving high-purity films at a consistent growth rate.
Common Pitfalls and Trade-offs
While powerful, the CVD process has inherent challenges that stem directly from its mechanism. Acknowledging these is essential for successful application.
Purity and Contamination
The process is highly sensitive to impurities. Any contaminants in the precursor gases or leaks in the vacuum chamber can become incorporated into the film, degrading its performance.
Uniformity Challenges
Achieving a perfectly even film thickness across a large or complex-shaped substrate is a significant challenge. It requires sophisticated control of temperature gradients and gas flow dynamics to ensure all surfaces receive an equal flux of reactants.
Precursor Selection
The choice of precursor is critical. An ideal precursor is volatile enough to be easily transported as a gas but stable enough not to decompose prematurely. Furthermore, its byproducts must be volatile and non-reactive to ensure they can be easily removed.
Making the Right Choice for Your Goal
The CVD mechanism can be tuned to achieve different outcomes. Your primary objective will determine which process parameters are most critical to control.
- If your primary focus is high-purity crystalline films: Prioritize ultra-pure precursor gases and precise, stable temperature control across the substrate.
- If your primary focus is coating complex shapes uniformly: Master the gas flow dynamics and pressure settings to ensure reactants are delivered evenly to all surfaces.
- If your primary focus is a high deposition rate: You will likely need to increase temperature and precursor concentration, but you must carefully manage this to avoid sacrificing film uniformity and purity.
By mastering the fundamental principles of this surface-catalyzed chemical reaction, you can effectively leverage the CVD process to engineer advanced materials with exceptional precision.
Summary Table:
| CVD Process Step | Key Action | Outcome |
|---|---|---|
| 1. Gas Introduction | Precursor gases enter the chamber | Volatile reactants are supplied |
| 2. Transport | Gases flow to the heated substrate | Molecules reach the surface for reaction |
| 3. Surface Reaction | Precursors adsorb and decompose on the substrate | Desired atoms are released for film formation |
| 4. Film Growth | Atoms nucleate and bond layer by layer | A solid, conformal coating is built up |
| 5. Byproduct Removal | Gaseous byproducts are swept away | High-purity, defect-free films are achieved |
Ready to achieve precise, high-purity thin films in your lab? KINTEK specializes in advanced CVD equipment and consumables, providing the reliable temperature control, gas delivery systems, and vacuum chambers needed to master the CVD mechanism. Whether you're coating complex geometries or growing crystalline materials, our solutions are engineered for superior uniformity and purity. Contact our experts today to discuss how we can optimize your deposition process!
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Laboratory CVD Boron Doped Diamond Materials
People Also Ask
- What is the difference between PECVD and APCVD? Choose the Right CVD Method for Your Application
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- What are different types of thin films? A Guide to Function, Material, and Deposition Methods
- What is the difference between plasma CVD and thermal CVD? Choose the Right Method for Your Substrate
- What are the process capabilities of ICPCVD systems? Achieve Low-Damage Film Deposition at Ultra-Low Temperatures



















