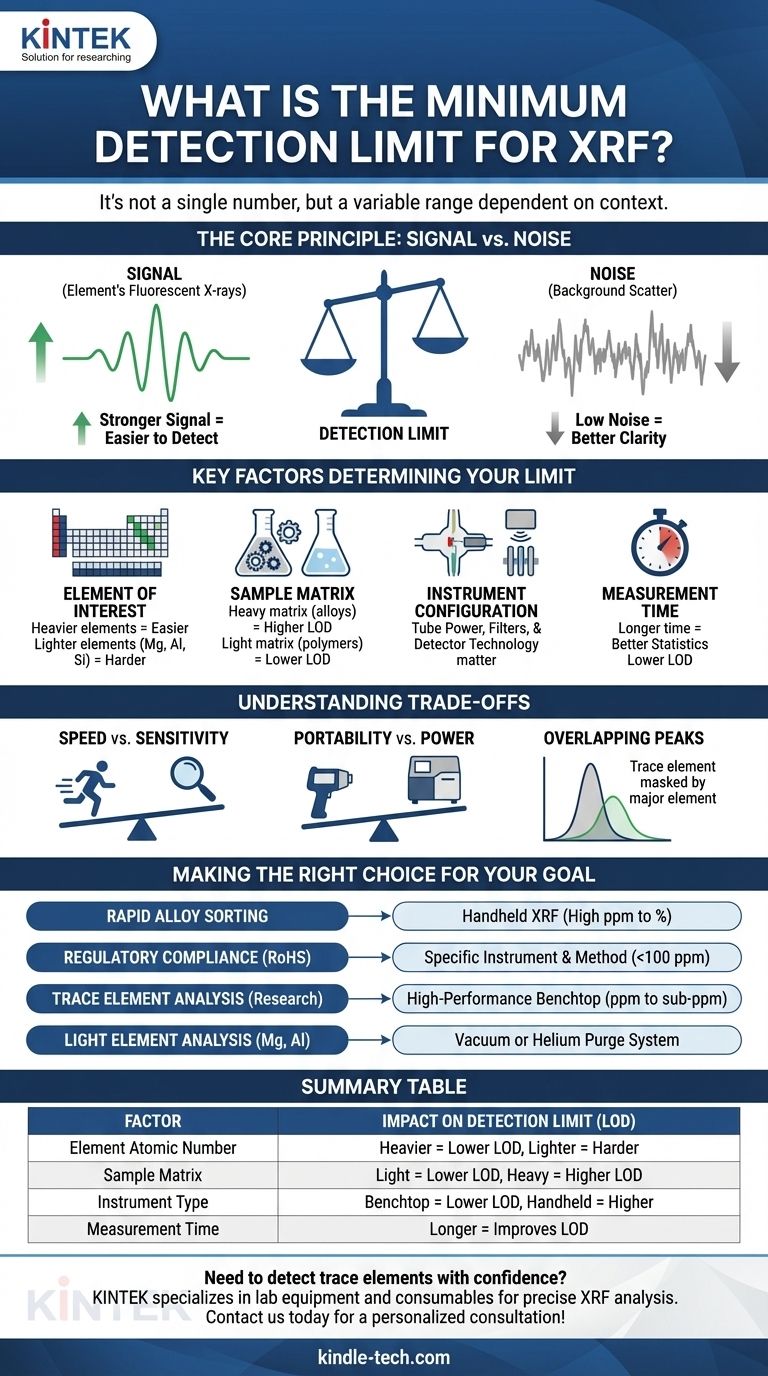
The minimum detection limit for X-ray Fluorescence (XRF) is not a single number, but a variable range that depends entirely on the context of the analysis. While it is possible to detect certain heavy elements in the low parts-per-million (ppm) range under ideal laboratory conditions, it is just as common for the limit to be in the hundreds of ppm or even percentage levels for lighter elements or in complex samples. The practical limit of detection (LOD) is a function of the element, the sample, and the instrument.
The most critical insight is to stop seeking a universal detection limit for XRF. Instead, the correct approach is to understand the factors that determine the achievable LOD for your specific element within your unique sample matrix, using a particular instrument configuration.

The Core Principle: Signal vs. Noise
At its heart, determining a detection limit is about one thing: reliably distinguishing the element's signal from the background noise. If the signal is too weak or the noise is too high, the element is undetectable.
What is the 'Signal'?
The signal is the count of characteristic fluorescent X-rays emitted by the atoms of your target element after being excited by the instrument's X-ray source. A stronger, more distinct signal is easier to detect.
What is the 'Noise'?
Noise is the background radiation that reaches the detector but is not from your target element. This primarily consists of scattered X-rays from the instrument's source that have reflected off the sample as a whole. A "clean" sample with low background noise makes it easier to see a weak signal.
Key Factors That Determine Your Detection Limit
Understanding why there is no single answer to the LOD question requires breaking down the variables that control the signal-to-noise ratio.
The Element of Interest
Heavier elements (those with a high atomic number, Z) are fundamentally easier to detect. They produce higher-energy X-rays that are less likely to be absorbed by the sample or the surrounding air.
Detecting light elements (like Magnesium, Aluminum, or Silicon) is much more challenging because their low-energy fluorescent X-rays are easily absorbed before they even reach the detector.
The Sample Matrix
The sample matrix refers to everything in the sample that is not the element you are trying to measure. This is often the most significant factor influencing detection limits.
A "heavy" matrix (like a metal alloy) will heavily absorb the signals from lighter elements within it, dramatically raising their detection limits. Conversely, a "light" organic matrix (like a polymer or oil) is more transparent to X-rays, resulting in lower detection limits for metals within it.
The Instrument Configuration
Different XRF analyzers have vastly different capabilities.
- X-ray Tube Power: Higher-power tubes (found in benchtop systems) generate a more intense primary beam, which in turn produces a stronger fluorescent signal from the sample, improving the LOD.
- Filters and Optics: Instruments use filters to "clean up" the source X-ray beam, removing parts of its spectrum that only contribute to background noise. This directly improves the signal-to-noise ratio for specific elemental groups.
- Detector Technology: Modern Silicon Drift Detectors (SDDs) offer better energy resolution and speed than older technologies. Better resolution allows the instrument to more clearly separate the X-ray peaks of different elements, which is crucial when one peak might otherwise hide another.
Measurement Time
This is a straightforward statistical variable. A longer measurement time allows the detector to collect more X-ray counts, which improves the statistical certainty of both the signal and the background. Doubling the measurement time does not halve the detection limit, but it will significantly improve it.
Understanding the Trade-offs
Choosing and using an XRF analyzer involves balancing competing priorities. Your LOD is directly affected by these choices.
Speed vs. Sensitivity
The most common trade-off is time. A 10-second "pass/fail" screening test will have a much higher (worse) detection limit than a deliberate 300-second analysis aimed at achieving the lowest possible LOD.
Portability vs. Power
A handheld XRF (pXRF) offers incredible convenience but has limitations in power and cooling. A laboratory-grade benchtop system (WDXRF or high-power EDXRF) provides a controlled environment, much higher power, and advanced optics, resulting in detection limits that can be 10 to 100 times lower than a handheld unit.
The Problem of Overlapping Peaks
In complex samples, a fluorescent peak from a major element can directly overlap with the peak of a trace element you are trying to measure. For example, the Arsenic (As) K-alpha peak is nearly identical in energy to the Lead (Pb) L-alpha peak. Detecting a few ppm of Arsenic in a sample containing thousands of ppm of Lead is extremely difficult, if not impossible, for XRF.
Making the Right Choice for Your Goal
To get a practical answer, you must define your analytical objective first.
- If your primary focus is rapid alloy sorting or material identification: A handheld XRF is ideal, and your concern is accurately measuring elements at percentage or high-ppm levels, well above typical detection limits.
- If your primary focus is regulatory compliance for heavy metals (e.g., RoHS, CPSIA): You need an instrument and method capable of reliably detecting elements like Lead, Cadmium, and Mercury well below the 100-1000 ppm legal thresholds.
- If your primary focus is trace element analysis for geology or research: You require a high-performance benchtop system, as you will be pushing the ppm and even sub-ppm boundaries where instrument stability and power are paramount.
- If your primary focus is analyzing light elements (Mg, Al, Si): You must use an instrument with a vacuum or helium purge system, as air completely absorbs their weak signals, making detection otherwise impossible.
By shifting your focus from a single number to the system of factors at play, you can confidently determine if XRF is the right tool for your analytical challenge.
Summary Table:
| Factor | Impact on Detection Limit (LOD) |
|---|---|
| Element Atomic Number | Heavier elements (e.g., Lead) have lower LODs; lighter elements (e.g., Magnesium) are harder to detect. |
| Sample Matrix | Light matrices (e.g., polymers) lower LODs; heavy matrices (e.g., metal alloys) raise LODs. |
| Instrument Type | Benchtop systems offer lower LODs (ppm to sub-ppm); handheld units are higher (100s of ppm). |
| Measurement Time | Longer analysis times improve LOD by increasing signal-to-noise ratio. |
Need to detect trace elements with confidence? KINTEK specializes in lab equipment and consumables, serving laboratory needs with precision XRF analyzers tailored for your specific application—whether for compliance (RoHS, CPSIA), research, or quality control. Our experts will help you select the right instrument to achieve the detection limits you require. Contact us today for a personalized consultation!
Visual Guide
Related Products
- Lab Plastic PVC Calender Stretch Film Casting Machine for Film Testing
- Laboratory Vibratory Sieve Shaker Machine for Dry and Wet Three-Dimensional Sieving
- Lab Blown Film Extrusion Three Layer Co-Extrusion Film Blowing Machine
- Single Punch Tablet Press Machine and Mass Production Rotary Tablet Punching Machine for TDP
- Laboratory Sterilizer Lab Autoclave Herbal Powder Sterilization Machine for Plant
People Also Ask
- What is the efficiency of a hydraulic press? Maximize Power and Performance for Your Applications
- What is KBr in chemistry? Discover Its Role in IR Spectroscopy and Beyond
- How does a laboratory hydraulic press contribute to g-CNT electrode preparation? Mastering Material Densification
- What is the difference between hydraulic and mechanical press used in forging? Choose the Right Press for Your Production Needs
- What are presses used for in manufacturing? The Ultimate Guide to Shaping Materials
- What are the characteristics and uses of a belt press in the HPHT process? Mastery of Industrial Diamond Synthesis
- What is the compression ratio of a pellet mill die? The Key to Durable, High-Quality Pellets
- What is the working of a hydraulic process? Harness Pascal's Law for Immense Force