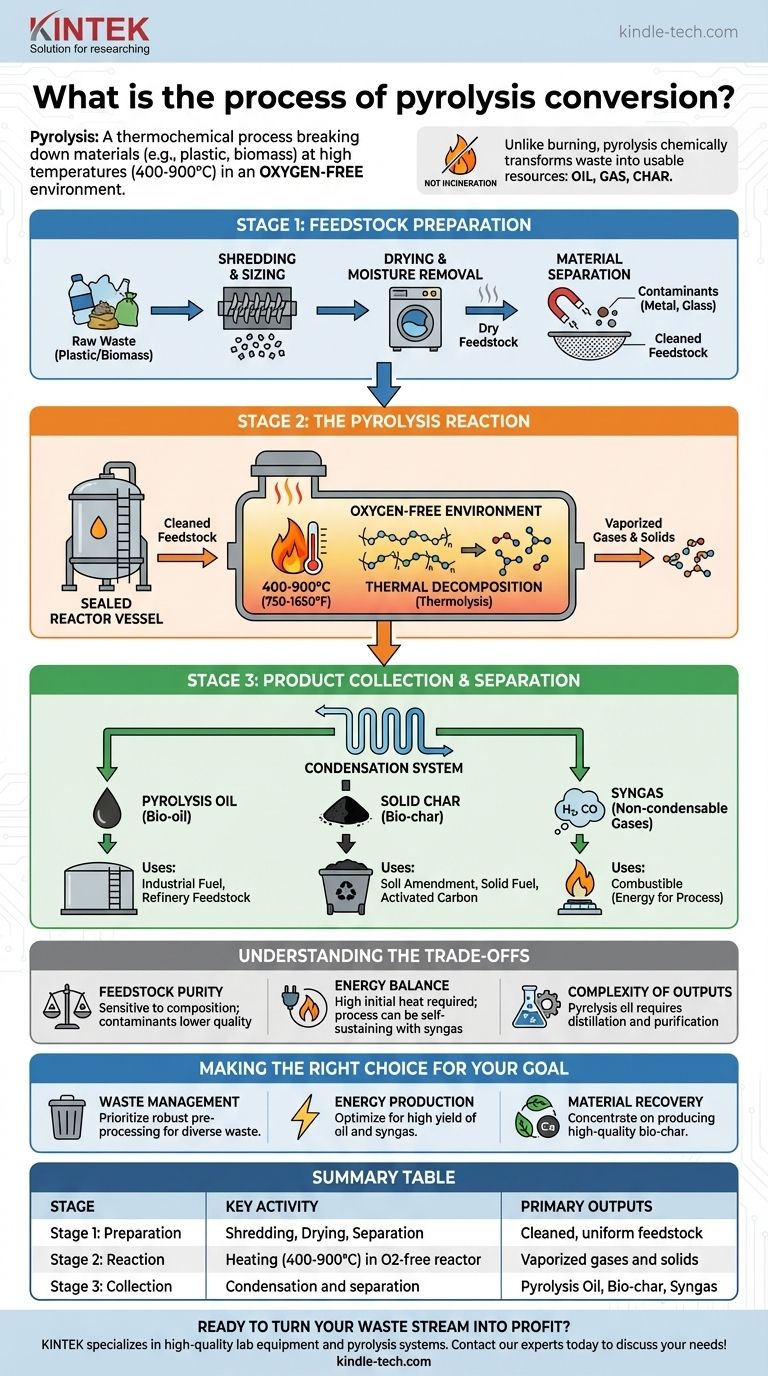
Pyrolysis conversion is a thermochemical process that breaks down materials, such as plastic or biomass, at high temperatures in an environment without oxygen. The core steps involve preparing the feedstock, heating it in a sealed reactor to trigger decomposition, and then collecting the resulting products: a liquid oil, a solid char, and a mixture of non-condensable gases.
Pyrolysis is not incineration or burning. By eliminating oxygen, it chemically decomposes materials into valuable new substances—oil, gas, and char—rather than simply combusting them into ash and flue gas, effectively transforming waste into a resource.

Stage 1: Feedstock Preparation
Before the main reaction can occur, the raw material must be properly prepared. This stage is critical for ensuring an efficient and clean conversion process.
Shredding and Sizing
The raw material, such as plastic waste or biomass, is first shredded into smaller, more uniform pieces. This increases the surface area, allowing heat to penetrate the material evenly and quickly during the reaction.
Drying and Moisture Removal
Excess moisture can hinder the pyrolysis process, consuming significant energy to evaporate and potentially creating undesirable byproducts. Feedstock is often dried to a specific moisture content for optimal performance.
Material Separation
For waste streams like mixed plastics, it is essential to separate non-pyrolyzable materials. Contaminants like metal, glass, or PVC are removed to prevent damage to the equipment and ensure the purity of the final products.
Stage 2: The Pyrolysis Reaction
This is the heart of the conversion process, where the prepared feedstock is chemically transformed.
The Oxygen-Free Reactor
The pre-processed material is fed into a sealed reactor vessel. All oxygen is purged from this chamber, which is the defining characteristic of pyrolysis. Without oxygen, the material cannot burn.
Applying High Heat
The reactor is heated to very high temperatures, typically between 400°C and 900°C (750°F to 1650°F). The precise temperature is controlled based on the type of feedstock and the desired output products.
Thermal Decomposition
The intense heat breaks down the long-chain polymer molecules of the feedstock into smaller, simpler molecules. This process is known as thermal decomposition or thermolysis. The material vaporizes into a mix of gases and solids.
Stage 3: Product Collection and Separation
As the hot vapor leaves the reactor, it is processed to separate it into distinct, usable products.
Pyrolysis Oil (Bio-oil)
The hot vapor is passed through a condensation system. The components that turn back into a liquid at room temperature are collected as pyrolysis oil (or bio-oil). This liquid can be purified and used as an industrial fuel or refined further.
Solid Char (Bio-char)
The solid, carbon-rich material that does not vaporize and remains in the reactor is known as bio-char. This substance can be used as a soil amendment to improve fertility, as a solid fuel, or as a feedstock for producing activated carbon.
Syngas
The gases that do not condense into oil are called non-condensable gases, or syngas. This mixture is typically rich in hydrogen and carbon monoxide and is combustible. It is often recycled to provide the heat needed to run the pyrolysis plant itself, making the process more energy-efficient.
Understanding the Trade-offs
Pyrolysis is a powerful technology, but its successful implementation depends on managing several key factors.
Feedstock Purity is Paramount
The process is highly sensitive to the composition of the input material. Contaminants can lower the quality of the outputs, damage the reactor, and increase the need for costly purification steps.
Energy Balance
While pyrolysis can be self-sustaining by burning its own syngas, the initial energy required to reach operating temperature is significant. The overall energy efficiency depends heavily on the scale of the operation and the moisture content of the feedstock.
Complexity of Outputs
Pyrolysis oil is not a drop-in replacement for crude oil; it is often acidic and unstable. It requires significant distillation and purification before it can be used in many applications, adding cost and complexity to the overall process.
Making the Right Choice for Your Goal
To apply pyrolysis effectively, align the process with your primary objective.
- If your primary focus is waste management: Prioritize robust pre-processing systems to handle diverse and potentially contaminated waste streams effectively.
- If your primary focus is energy production: Optimize the process for a high yield of pyrolysis oil and syngas, which can be used to generate heat or power.
- If your primary focus is material recovery: Concentrate on producing a high-quality bio-char for applications like soil amendment or activated carbon production.
Understanding these core stages and their variables empowers you to evaluate and implement pyrolysis technology to meet your specific objective.
Summary Table:
| Pyrolysis Stage | Key Activity | Primary Outputs |
|---|---|---|
| Stage 1: Feedstock Preparation | Shredding, Drying, Material Separation | Cleaned, uniform feedstock |
| Stage 2: Pyrolysis Reaction | Heating in oxygen-free reactor (400-900°C) | Vaporized gases and solids |
| Stage 3: Product Collection | Condensation and separation | Pyrolysis Oil, Bio-char, Syngas |
Ready to turn your waste stream into profit? KINTEK specializes in high-quality lab equipment and pyrolysis systems for efficient material conversion. Whether your goal is waste management, energy production, or material recovery, our solutions are designed to maximize your output of valuable pyrolysis oil, bio-char, and syngas. Contact our experts today to discuss how a KINTEK pyrolysis system can be tailored to your specific laboratory or industrial needs!
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Vertical Laboratory Tube Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What are the equipment requirements for loading platinum (Pt) onto composite supports? Precise Stirring for High Dispersion
- What temperature is needed for pyrolysis waste? A Guide to Optimizing Your Waste-to-Value Process
- How do high-temperature reaction furnaces control in-situ MMCs? Master Material Precision and Structural Integrity
- What are the process advantages of using a rotary tube furnace for WS2 powder? Achieve Superior Material Crystallinity
- What is the difference between pyrolysis combustion and gasification? A Guide to Thermal Conversion Technologies



















