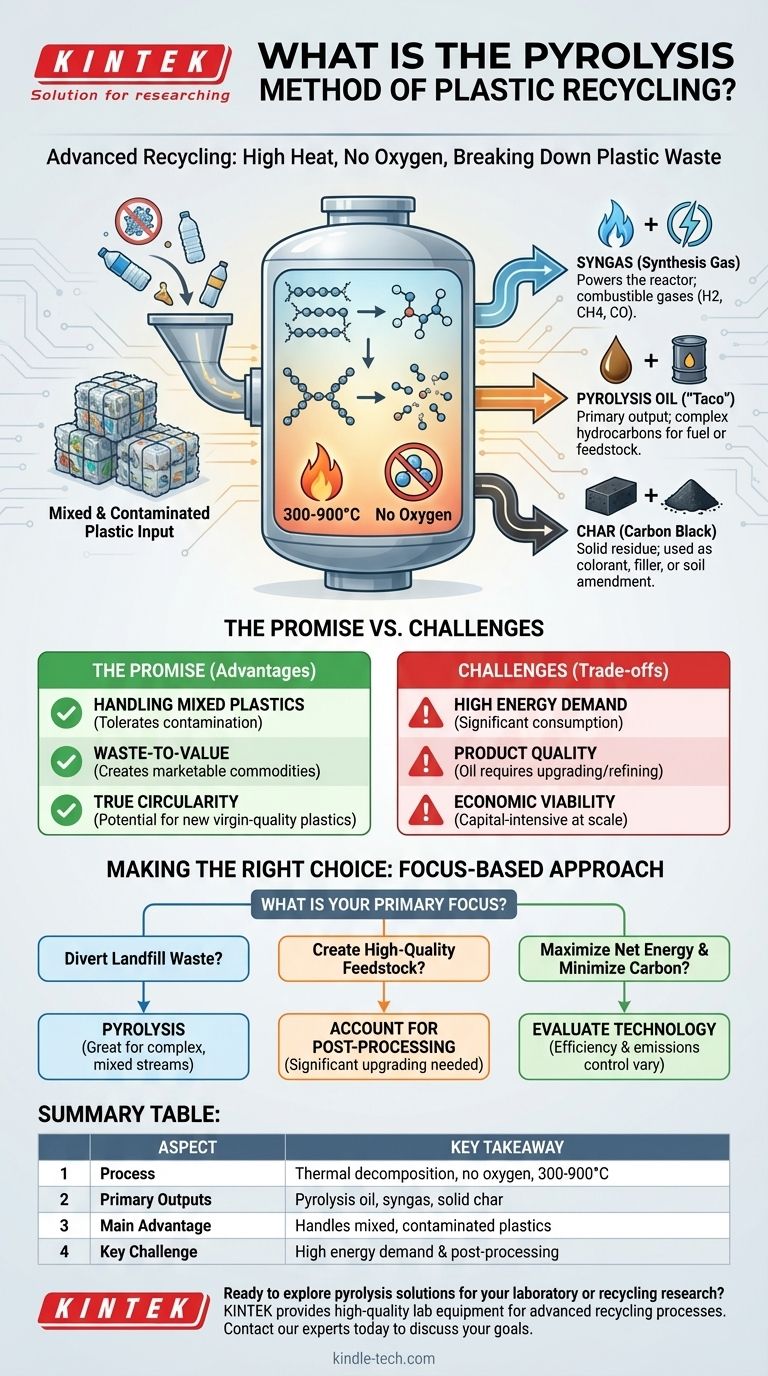
At its core, pyrolysis is a form of advanced recycling that uses high heat in an oxygen-free environment to break down plastic waste. Instead of burning the material, this process thermally decomposes long plastic polymer chains into simpler, valuable substances, primarily a synthetic oil, a combustible gas, and a solid char.
Pyrolysis offers a compelling solution for converting hard-to-recycle plastics into new resources, moving beyond the limitations of traditional methods. However, its effectiveness is not guaranteed; it hinges on the efficiency of the specific process, its energy demands, and the quality of the final products.

How Pyrolysis Works: A Look Inside the Reactor
Pyrolysis is fundamentally a chemical decomposition process driven by heat. It functions by reversing the original polymerization process, breaking down large, complex molecules into smaller, simpler ones.
The Critical Role of Heat
Plastics are fed into a reactor vessel and heated to extreme temperatures, typically between 300°C and 900°C (570°F to 1650°F). This intense heat provides the energy needed to sever the strong chemical bonds that hold the long polymer chains together.
The Absence of Oxygen
This is the key element that distinguishes pyrolysis from incineration (burning). By removing oxygen from the reactor, we prevent combustion. Instead of burning and releasing its energy as heat and smoke, the plastic "cracks" into smaller hydrocarbon molecules.
The Three Primary Outputs
The process separates the decomposed plastic into three distinct product streams.
- Pyrolysis Oil (or "Taco"): This liquid product, technically known as "plastic-derived pyrolysis oil," is the primary output. It is a complex mixture of hydrocarbons that can be refined and upgraded for use as fuel or as a feedstock to create new chemicals and even new plastics.
- Syngas (Synthesis Gas): This is a mixture of non-condensable, combustible gases like hydrogen, methane, and carbon monoxide. In most modern facilities, this syngas is captured and used to power the pyrolysis reactor itself, helping to offset the process's high energy requirements.
- Char (or Carbon Black): This solid, carbon-rich residue is what remains after the volatile components have been driven off. Depending on the purity of the input plastic, this char can be used as a colorant, a filler material, or a soil amendment, though it may also contain contaminants that require safe disposal.
The Promise: Why Consider Pyrolysis?
Pyrolysis addresses several of the core weaknesses inherent in traditional mechanical recycling, where plastic is shredded, washed, and melted down.
Handling Contaminated and Mixed Plastics
Mechanical recycling requires extremely clean and well-sorted plastics. Pyrolysis is far more tolerant. It can process mixed plastic bales, multi-layer films, and plastics contaminated with food residue or paper labels that would otherwise be sent to a landfill.
Creating New Value from Waste
This technology embodies the "waste-to-value" principle. It can take a low-value or negative-value material (waste plastic) and convert it into marketable commodities like synthetic oil and chemical feedstocks, creating economic incentives for waste collection.
Potential for True Circularity
When pyrolysis oil is refined and used to create new "virgin-quality" plastics, it enables a true closed-loop system. Unlike mechanical recycling, which often "downcycles" plastic into lower-quality products, this chemical recycling path can theoretically repeat indefinitely without degrading material quality.
Understanding the Trade-offs and Challenges
While promising, pyrolysis is not a perfect solution. It presents its own set of technical, economic, and environmental challenges that must be carefully managed.
High Energy Demand
Bringing a reactor to the required temperatures and keeping it there consumes a significant amount of energy. The overall net energy benefit of the process is a critical factor; an inefficient plant may consume more energy than it produces, undermining its environmental advantages.
The Quality of the End Products
Pyrolysis oil is not equivalent to fossil crude oil. It is often acidic, unstable, and contains impurities from additives and contaminants in the original plastic waste. It requires significant, energy-intensive pre-treatment and upgrading before it can be used in a conventional refinery or chemical plant.
Emissions and Environmental Risk
While pyrolysis avoids the direct smokestack emissions of incineration, it is not without environmental risks. Poor process control or air leaks can lead to the formation and release of hazardous pollutants. The overall carbon footprint, including transportation and refining, must be compared against virgin production and landfilling.
Economic Viability at Scale
Pyrolysis facilities are capital-intensive to build and operate. The economic success of a plant depends heavily on the price of its outputs (oil, char), the cost of its inputs (waste plastic, energy), and the reliability of its technology. Achieving profitability at a large, industrial scale remains a significant hurdle.
Making the Right Choice for Your Goal
Pyrolysis is best viewed as a specialized tool for a specific set of problems within the broader waste management landscape. Its suitability depends entirely on the intended outcome.
- If your primary focus is diverting complex, non-recyclable plastic waste from landfills: Pyrolysis is one of the most promising technologies available, as it can handle mixed and contaminated streams that other systems cannot.
- If your primary focus is creating high-quality fuel or chemical feedstock: You must account for the significant post-processing and upgrading required to convert raw pyrolysis oil into a usable, refinery-ready product.
- If your primary focus is maximizing net energy production and minimizing carbon footprint: Critically evaluate the specific technology's energy balance and emissions control systems, as efficiency varies dramatically between different vendors and designs.
Ultimately, pyrolysis is a powerful method for unlocking the value trapped in plastic waste, but its successful implementation demands a clear-eyed understanding of its technical complexities and economic trade-offs.
Summary Table:
| Aspect | Key Takeaway |
|---|---|
| Process | Thermal decomposition of plastic in an oxygen-free environment at 300-900°C. |
| Primary Outputs | Pyrolysis oil (fuel/feedstock), syngas (process fuel), solid char (filler/colorant). |
| Main Advantage | Handles mixed, contaminated plastics that mechanical recycling cannot. |
| Key Challenge | High energy demand and need for post-processing of the oil output. |
Ready to explore pyrolysis solutions for your laboratory or recycling research?
KINTEK specializes in providing high-quality lab equipment and consumables for advanced recycling processes like pyrolysis. Whether you are conducting R&D on process optimization, product upgrading, or emissions analysis, our reliable equipment can support your critical work in converting plastic waste into valuable resources.
Contact our experts today to discuss how our solutions can help you achieve your research and sustainability goals.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
People Also Ask
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success



















