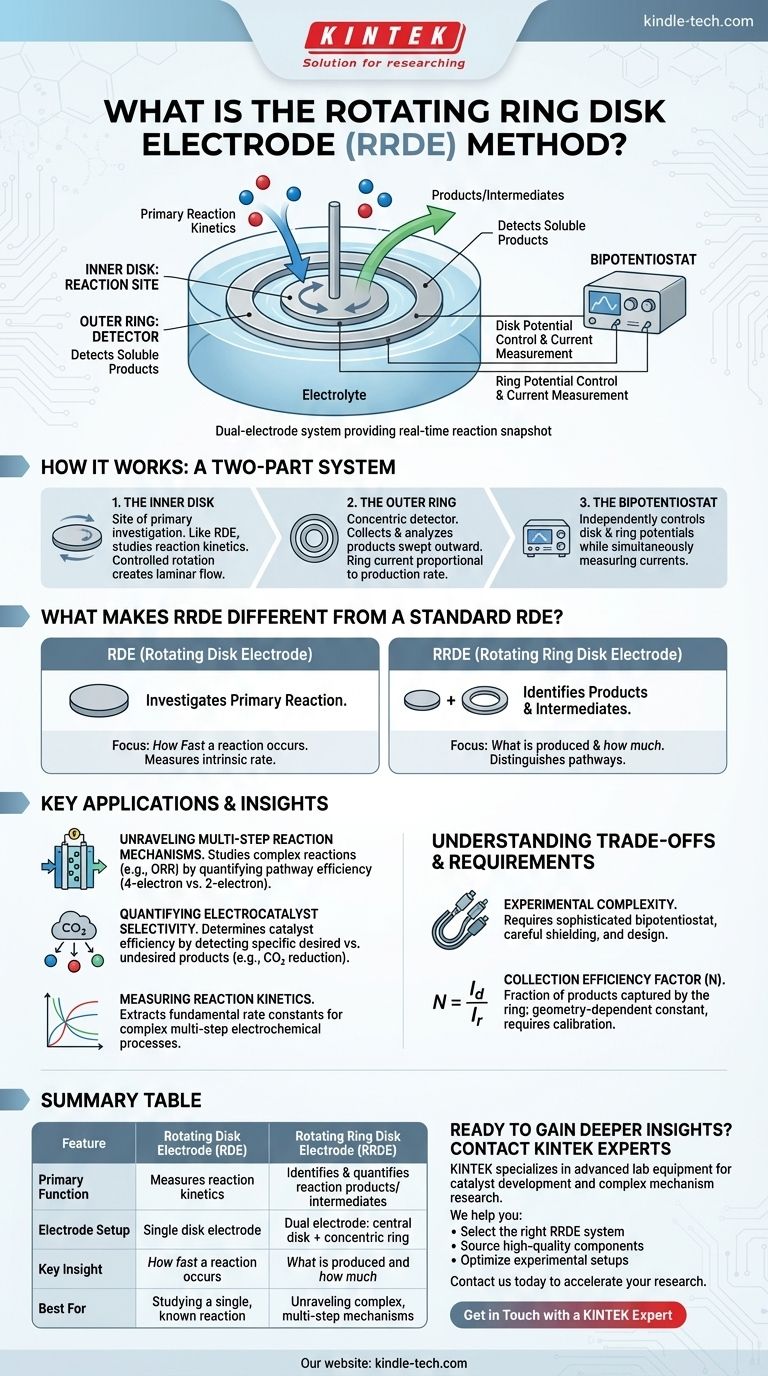
The rotating ring disk electrode (RRDE) method is an advanced electrochemical technique that uses a dual-electrode setup—a central disk surrounded by a concentric ring—to simultaneously drive a reaction and detect its products. While a reaction is initiated at the spinning disk, the ring electrode is set at a different potential to capture and analyze any chemical species that are generated and transported outward from the disk.
The core value of the RRDE is its ability to provide a "snapshot" of a reaction's output in real time. It moves beyond simply measuring the primary reaction's kinetics to actively identifying the products and intermediates, which is critical for understanding complex electrochemical mechanisms.

How the RRDE Works: A Two-Part System
The RRDE's power comes from its unique geometry and the controlled hydrodynamics created by its rotation. It is essentially two independent working electrodes functioning in a highly coordinated manner.
The Inner Disk: The Reaction Site
The central disk is the primary site of investigation. It functions like a standard rotating disk electrode (RDE), where the electrochemical reaction of interest occurs.
The entire assembly is attached to a motor that spins it at a precise and controllable rate. This rotation pulls the electrolyte solution towards the electrode and then forces it radially outward across the surface in a well-defined, laminar flow.
The Outer Ring: The Detector
Surrounding the disk is a concentric, electrically isolated ring. This ring acts as a detector for any soluble products or intermediates generated at the disk.
As these species are swept outward by the fluid flow, a fraction of them passes over the ring. By setting the ring to an appropriate potential, these species can be oxidized or reduced, generating a current at the ring that is directly proportional to their production rate at thedisk.
The Bipotentiostat: Dual Control
To operate this two-electrode system, a bipotentiostat is required. This instrument allows an electrochemist to control the potential of the disk and the ring independently while simultaneously measuring the current at both.
What Makes RRDE Different from a Standard RDE?
While closely related, the Rotating Disk Electrode (RDE) and RRDE answer fundamentally different questions.
RDE: Investigating the Primary Reaction
A standard RDE is used to study the kinetics of a single reaction occurring at the disk. By varying the rotation rate, one can control the mass transport of reactants to the surface and isolate the reaction's intrinsic rate. It tells you how fast a reaction is happening.
RRDE: Identifying Reaction Products and Intermediates
The RRDE does everything an RDE does, but adds a crucial second layer of information. The ring current tells you what is being produced at the disk and how much. This is indispensable for identifying unstable intermediates and distinguishing between competing reaction pathways.
Key Applications and Insights Gained
The ability to correlate the reaction at the disk with the detection of products at the ring makes RRDE a powerful tool for mechanistic studies.
Unraveling Multi-Step Reaction Mechanisms
RRDE is a classic tool for studying reactions like the oxygen reduction reaction (ORR). It can precisely quantify whether the reaction proceeds directly to water (a 4-electron pathway) or produces hydrogen peroxide as an intermediate (a 2-electron pathway), which is critical for fuel cell catalyst development.
Quantifying Electrocatalyst Selectivity
In areas like CO₂ reduction, where multiple products can be formed, the RRDE can help determine the efficiency and selectivity of a catalyst. The ring can be set to detect specific products, revealing how effectively the disk catalyst converts a reactant into a desired chemical.
Measuring Reaction Kinetics
By analyzing the relationship between the disk and ring currents at various rotation speeds, researchers can extract fundamental kinetic rate constants for complex, multi-step electrochemical processes.
Understanding the Trade-offs and Requirements
While powerful, the RRDE method requires a more sophisticated setup and data interpretation than a standard RDE experiment.
Experimental Complexity
The use of a bipotentiostat and the need to manage two independent electrode processes adds a layer of complexity to the experiment. Proper shielding and careful cell design are crucial to avoid electrical interference between the disk and ring signals.
The Collection Efficiency Factor
Not all of the products generated at the disk will be intercepted by the ring; some will diffuse away into the bulk solution. The fraction that is captured is known as the collection efficiency (N).
This is a constant value determined purely by the electrode's geometry. It must be known or calibrated beforehand to accurately quantify the products generated at the disk from the current measured at the ring.
Making the Right Choice for Your Goal
Selecting the correct hydrodynamic technique depends entirely on the question you need to answer.
- If your primary focus is studying the overall kinetics of a single, known reaction: A standard Rotating Disk Electrode (RDE) is often sufficient and simpler to implement.
- If your primary focus is distinguishing between multiple reaction pathways: The RRDE is the superior tool, as it allows you to detect the formation of specific intermediates or side products.
- If your primary focus is evaluating the selectivity and efficiency of a new electrocatalyst: The RRDE is essential for quantifying the production of desired vs. undesired products.
Ultimately, the RRDE provides a deeper, more mechanistic understanding of electrochemical processes by directly linking an initial reaction to its chemical output.
Summary Table:
| Feature | Rotating Disk Electrode (RDE) | Rotating Ring Disk Electrode (RRDE) |
|---|---|---|
| Primary Function | Measures reaction kinetics | Identifies & quantifies reaction products/intermediates |
| Electrode Setup | Single disk electrode | Dual electrode: central disk + concentric ring |
| Key Insight | How fast a reaction occurs | What is produced and how much |
| Best For | Studying a single, known reaction | Unraveling complex, multi-step mechanisms |
Ready to gain deeper insights into your electrochemical reactions?
The Rotating Ring Disk Electrode (RRDE) method is essential for researchers and labs focused on catalyst development, fuel cell research, and complex reaction mechanisms. KINTEK specializes in providing the advanced lab equipment and consumables you need to implement these sophisticated techniques effectively.
We can help you:
- Select the right RRDE system for your specific application.
- Source high-quality electrodes and bipotentiostats for reliable, accurate data.
- Optimize your experimental setup to study reaction pathways and catalyst selectivity with confidence.
Contact us today using the form below to discuss how our solutions can accelerate your research and deliver the mechanistic understanding you need.
Get in Touch with a KINTEK Expert
Visual Guide
Related Products
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Metal Disc Electrode Electrochemical Electrode
- Graphite Disc Rod and Sheet Electrode Electrochemical Graphite Electrode
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
People Also Ask
- What is the RRDE in electrochemistry? Unlock Detailed Reaction Pathways with Dual-Electrode Analysis
- What role does the RRDE play in catalyst evaluation for H2O2 synthesis? Enhance Selectivity and Kinetic Precision
- Why is a high-precision Rotating Ring-Disk Electrode (RRDE) essential for ORR? Unlock Precise Catalytic Kinetics
- What is the function of a Laboratory RDE system for OER catalysts? Optimize Kinetic Activity Screening
- What is the difference between ring disk electrode and rotating disk electrode? Unlock Deeper Electrochemical Insights



















