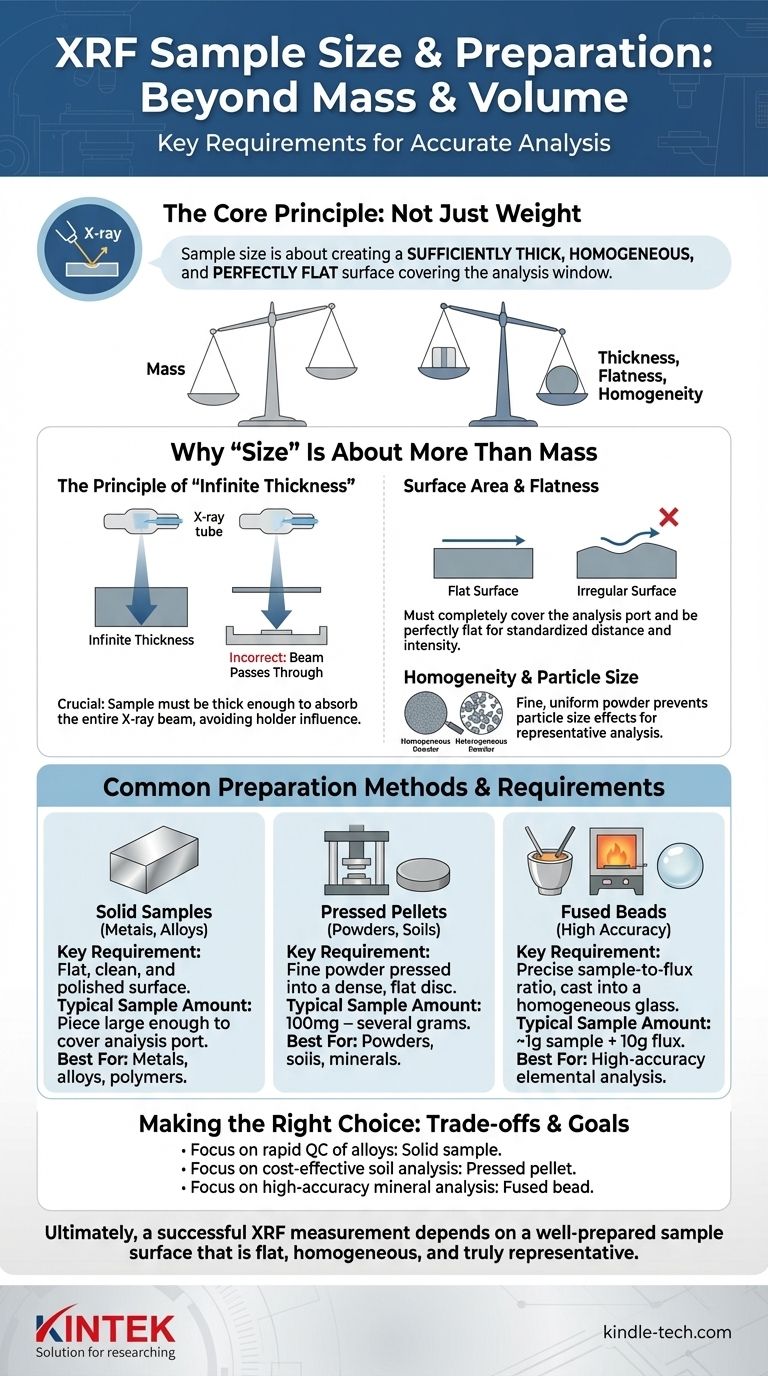
For X-Ray Fluorescence (XRF) analysis, there is no single, universal sample size. The primary requirement is not a specific mass or volume, but rather creating a sample that is sufficiently thick, homogeneous, and presents a perfectly flat surface that completely covers the instrument's analysis window. The necessary amount of material depends entirely on the sample type and the preparation method used to meet these conditions.
The core principle of XRF sample preparation is not to meet a weight requirement, but to create a sample that is "infinitely thick" to the X-ray beam. This ensures the analysis is representative of the material itself, not influenced by the sample holder or inconsistent thickness.

Why "Size" Is About More Than Mass
In XRF, the instrument's X-ray tube irradiates a specific area on the sample's surface. The detector then measures the secondary X-rays emitted from that spot. This surface-sensitive nature means the geometry and consistency of the sample are far more critical than its total weight.
The Principle of "Infinite Thickness"
The most crucial concept is ensuring your sample is "infinitely thick." This does not mean the sample is physically enormous.
It means the sample is thick enough that the primary X-ray beam is completely absorbed within the material. None of the beam should pass through to the sample holder underneath, as this would introduce errors and inaccurate results.
The Importance of Surface Area and Flatness
The sample must be wide enough to completely cover the analysis port of the spectrometer. Any gaps will lead to invalid readings.
Furthermore, the surface must be perfectly flat. As noted in technical calibrations, XRF systems are standardized for a fixed distance between the X-ray source and the sample. An irregular, bumpy, or curved surface alters this distance, which changes the intensity of the detected elements and compromises the accuracy of your results.
Homogeneity and Particle Size Effects
For powders, soils, or minerals, the sample must be homogeneous. The small area being analyzed must be perfectly representative of the entire bulk material.
To achieve this, samples are typically ground into a fine powder (often less than 75 µm). If coarse or uneven particles are present, the analysis may disproportionately measure one particle type over another, an error known as the "particle size effect."
Common Preparation Methods and Their Requirements
The amount of raw material you need is dictated by the method used to create a suitable analytical surface.
Solid Samples (e.g., Metals, Alloys, Polymers)
For a solid, uniform piece of metal or plastic, the "sample size" is simply a piece large enough to present a flat, clean, and polished surface to the instrument. The total mass is irrelevant. Preparation involves machining or polishing a representative surface.
Powdered Samples (Pressed Pellets)
This is a very common method for powders, minerals, and soils. The material is ground finely and pressed under high pressure into a dense, flat disc (pellet).
The amount of sample needed is whatever is required to form a robust pellet that is "infinitely thick." This typically ranges from a few hundred milligrams to several grams, depending on the density of the material and the diameter of the pellet die (e.g., 32mm or 40mm).
Fused Beads
For the highest accuracy, powders are often mixed with a flux (like a lithium borate salt) and heated in a crucible until molten. The molten glass is then cast into a perfectly flat and homogeneous disc.
This method requires a precise ratio of sample to flux. The "sample size" is therefore a specific, smaller amount (e.g., 1 gram of sample to 10 grams of flux) calculated to ensure homogeneity while avoiding over-dilution.
Understanding the Trade-offs
Choosing a preparation method involves balancing speed, cost, and the quality of results you need.
Pressed Pellets vs. Fused Beads
Pressed pellets are fast, inexpensive, and preserve the original sample concentration, which is good for trace element analysis. However, they can suffer from particle size and mineralogical effects that reduce accuracy if not prepared carefully.
Fused beads eliminate particle size effects entirely, creating a nearly perfect sample surface that yields highly accurate and repeatable results. The main trade-offs are the higher complexity, longer preparation time, and the dilution of the sample, which can make it harder to detect trace elements.
The Risk of Contamination
During any preparation step, contamination is a critical risk. When preparing solid metals, separate files or polishing pads must be used for different alloy types. When grinding powders, the grinding vessel itself can introduce contaminants (e.g., tungsten carbide from the mill) that will be detected by the XRF.
Making the Right Choice for Your Goal
Your sample preparation strategy should be guided by your analytical objective.
- If your primary focus is rapid quality control of a solid alloy: Ensure you have a piece large enough to machine or polish a flat, representative surface.
- If your primary focus is high-accuracy elemental analysis of a mineral: Use the fused bead method, accepting the dilution in exchange for superior precision and the elimination of physical effects.
- If your primary focus is cost-effective analysis of soils or powders: Use the pressed pellet method, but ensure the material is ground finely and uniformly to create a thick, homogeneous pellet.
Ultimately, a successful XRF measurement depends not on a specific sample weight, but on a well-prepared sample surface that is flat, homogeneous, and truly representative of your material.
Summary Table:
| Preparation Method | Key Requirement | Typical Sample Amount | Best For |
|---|---|---|---|
| Solid Samples | Flat, polished surface | Piece large enough to cover analysis port | Metals, alloys, polymers |
| Pressed Pellets | Fine powder, homogeneous | 100mg - several grams | Powders, soils, minerals |
| Fused Beads | Precise sample-to-flux ratio | ~1g sample + 10g flux | High-accuracy elemental analysis |
Struggling with inconsistent XRF results? Your sample preparation is likely the key. At KINTEK, we specialize in laboratory equipment and consumables for precise XRF analysis. Whether you need reliable pellet presses for consistent powder preparation or guidance on choosing the right method for your materials, our experts can help you achieve accurate, repeatable results.
Let KINTEK optimize your XRF workflow. Contact our team today to discuss your specific application and ensure your samples are prepared for success.
Visual Guide
Related Products
- Laboratory Hydraulic Pellet Press for XRF KBR FTIR Lab Applications
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Laboratory Manual Hydraulic Pellet Press for Lab Use
People Also Ask
- Why is a hydraulic press used for pre-deformation treatment? Enhance Coating Hardness & Thermal Stability
- What is an automatic press machine? High-Precision Force for Modern Manufacturing
- What affects the speed of a hydraulic motor? Master the balance of flow rate and displacement
- How does a power press work? Unlock the Power of Mechanical and Hydraulic Presses
- What role does a high-precision laboratory hydraulic press play in ICDP membranes? Optimize Ceramic Support Formation
- What role does a uniaxial hydraulic press play in the fabrication of NaSICON ceramic cylinders? Pre-forming Excellence
- How do laboratory hydraulic presses and specialized fixtures ensure the accuracy of electrochemical testing? (Expert Guide)
- Why is a laboratory hydraulic press preferred over sintering for sulfide electrolyte anode frameworks? (LPS)



















