At its core, pyrolysis is powered by external thermal energy. The process requires a significant input of heat to break down organic material in an oxygen-free environment. While pyrolysis does generate energy-rich products, it is not a spontaneous reaction and needs an external source to initiate and sustain it.
The central concept to grasp is that pyrolysis consumes heat to operate, but it can be engineered to become self-sustaining by using a portion of the combustible gases it produces as its own fuel source.

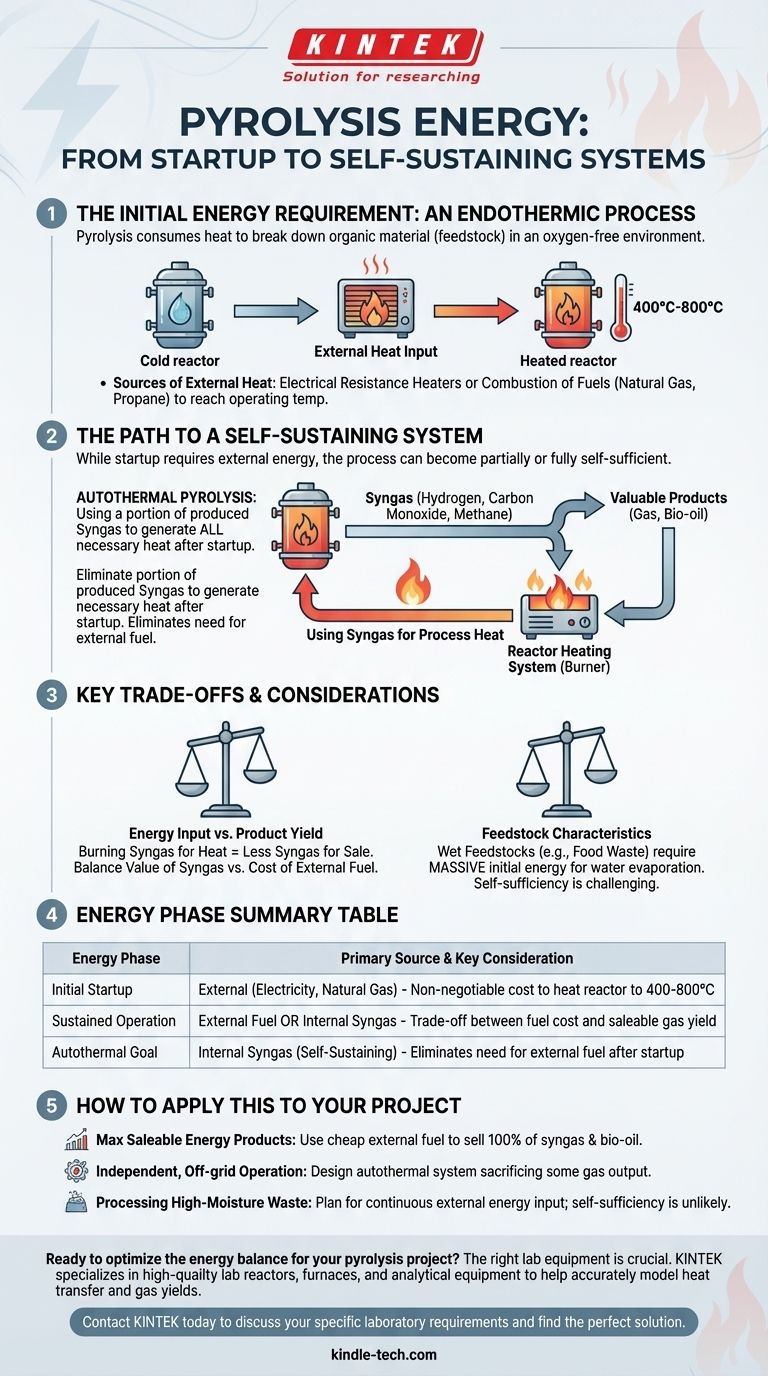
The Initial Energy Requirement: An Endothermic Process
Pyrolysis is fundamentally an endothermic process, meaning it requires a continuous input of energy to break the strong chemical bonds within the feedstock. Without a consistent source of heat, the reaction would simply stop.
Sources of External Heat
To start the process and bring the reactor to the required operating temperature (typically between 400°C and 800°C), operators rely on conventional energy sources. These most commonly include electrical resistance heaters or the combustion of fuels like natural gas or propane.
The Role of Heat Transfer
This initial energy is transferred to the feedstock through conduction, convection, or radiation. The efficiency of this heat transfer is a critical factor in the overall energy consumption of the pyrolysis system.
The Path to a Self-Sustaining System
While pyrolysis requires external energy to start, many systems are designed to become partially or fully self-sufficient once they are running at a steady state.
Using Syngas for Process Heat
Pyrolysis produces a mixture of non-condensable gases known as synthesis gas (syngas). This gas is rich in components like hydrogen, carbon monoxide, and methane, making it highly combustible.
A common and efficient design strategy is to redirect a portion of this syngas back to the reactor's heating system. By burning its own gaseous byproduct, the system can provide the necessary heat to sustain the pyrolytic reaction.
The Concept of Autothermal Pyrolysis
When a system is designed to use its own syngas to generate all the heat it needs after the initial startup phase, it is referred to as autothermal. In this state, the need for external fuel (like natural gas) is eliminated, dramatically improving the process's net energy balance and operational cost.
Understanding the Trade-offs
Achieving a self-sustaining pyrolysis process involves critical engineering and economic trade-offs that determine the overall viability of a project.
Energy Input vs. Product Yield
The most significant trade-off is clear: any syngas burned to heat the reactor is syngas that cannot be sold or used for other valuable purposes, such as generating electricity or synthesizing chemicals. The decision depends on the relative value of the syngas versus the cost of external fuel.
Feedstock Characteristics Matter
The energy balance is highly dependent on the feedstock. Wet feedstocks, like food waste or sewage sludge, require a massive amount of initial energy to evaporate water before pyrolysis can even begin. For these materials, achieving self-sufficiency is far more challenging, and a continuous external energy source is often necessary.
Startup Energy is a Fixed Cost
Even in a fully autothermal system, the initial energy required to bring the cold reactor up to operating temperature is a non-negotiable cost. This startup phase represents a significant energy investment for any pyrolysis operation.
How to Apply This to Your Project
Understanding the energy source is fundamental to designing a successful pyrolysis system. Your choice will depend entirely on your primary objective.
- If your primary focus is maximizing saleable energy products: You might opt to power the process with a cheap, external fuel source, which allows you to capture and sell 100% of the valuable syngas and bio-oil produced.
- If your primary focus is creating an independent, off-grid operation: You will design an autothermal system that sacrifices a portion of its gas output to eliminate reliance on external fuel infrastructure.
- If your primary focus is processing high-moisture waste: You must plan for a significant and continuous energy input, as achieving self-sufficiency with wet feedstock is often not feasible.
Ultimately, mastering the energy balance of pyrolysis is the key to moving from a theoretical concept to an economically and environmentally viable solution.
Summary Table:
| Energy Phase | Primary Source | Key Consideration |
|---|---|---|
| Initial Startup | External (Electricity, Natural Gas) | Non-negotiable cost to heat reactor to 400-800°C |
| Sustained Operation | External Fuel or Internal Syngas | Trade-off between fuel cost and saleable gas yield |
| Autothermal Goal | Internal Syngas (Self-Sustaining) | Eliminates need for external fuel after startup |
Ready to optimize the energy balance for your pyrolysis project? The right lab equipment is crucial for testing feedstock and designing an efficient system. KINTEK specializes in high-quality lab reactors, furnaces, and analytical equipment to help you accurately model heat transfer and gas yields. Whether your goal is maximizing product output or achieving off-grid operation, our experts can provide the tools and support you need. Contact KINTEK today to discuss your specific laboratory requirements and find the perfect solution for your research and development.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
People Also Ask
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas



















