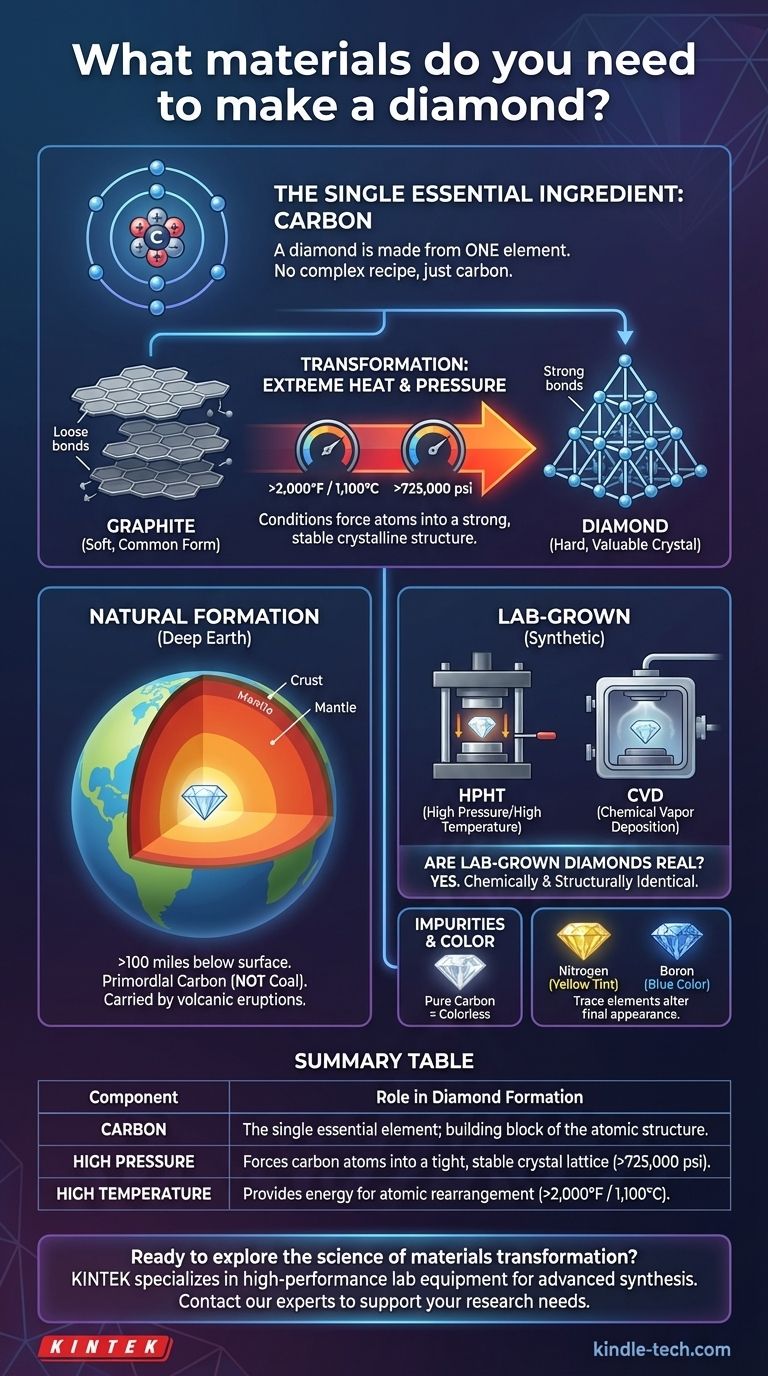
At its core, a diamond is made from a single, surprisingly common material. The only element required to create a diamond is carbon. The true magic lies not in a complex recipe of materials, but in subjecting this one element to immense heat and pressure, which forces its atoms into an incredibly strong and stable crystalline structure.
The essential takeaway is that you don't mix materials to make a diamond. You take one material—carbon—and subject it to extraordinary pressure and temperature to fundamentally change its atomic structure from a common form like graphite into the valuable crystal we know as diamond.

The Single, Essential Ingredient: Carbon
To understand how a diamond is made, we must first focus on its sole component. Everything about a diamond begins and ends with the element carbon.
Carbon's Two Faces: Graphite vs. Diamond
Carbon is a versatile element capable of forming different structures, known as allotropes. The soft, gray graphite in a pencil and the hard, transparent diamond in a ring are both 100% pure carbon.
The difference is not the material, but the arrangement of the carbon atoms. The conditions under which the carbon crystallizes determine its final form and properties.
The Myth of Compressed Coal
A common misconception is that diamonds are formed from the compression of coal. This is scientifically inaccurate.
Most natural diamonds are far older than the Earth's first land plants, which are the source material for coal. The carbon source for most diamonds is primordial, trapped deep within the Earth's mantle since the planet's formation.
The Two Critical Conditions: Heat and Pressure
While carbon is the what, extreme heat and pressure are the how. These two conditions are the forces that forge a diamond's unique structure.
How Natural Diamonds Form
Natural diamonds are created deep within the Earth's mantle, typically more than 100 miles below the surface. Here, temperatures exceed 2,000°F (1,100°C) and pressures are over 725,000 pounds per square inch.
Under this immense force and heat, carbon atoms are forced into a tight, rigid, three-dimensional lattice. These diamonds are then carried rapidly to the surface by deep-source volcanic eruptions.
Simulating Nature: Lab-Grown Diamonds
Scientists can replicate these conditions in a lab to create synthetic diamonds that are chemically and physically identical to natural ones.
The two primary methods are High Pressure/High Temperature (HPHT), which mimics the mantle's conditions, and Chemical Vapor Deposition (CVD), which "grows" a diamond layer by layer from a carbon-rich gas. In both cases, the only essential material is a source of pure carbon.
Common Pitfalls and Misconceptions
Understanding the truth about diamond creation requires navigating a few common but persistent myths. Clarity on these points is crucial for a true understanding.
Are Lab-Grown Diamonds "Real"?
Yes. A lab-grown diamond is not a "fake" diamond like cubic zirconia. It is structurally and chemically a real diamond.
The only difference is its origin. Since it is composed of carbon atoms arranged in the same cubic lattice, it possesses the same hardness, brilliance, and thermal conductivity as a diamond formed in the Earth.
The Role of Impurities
While a perfect diamond is pure carbon, tiny amounts of other elements trapped during its formation can introduce color.
For example, nitrogen atoms can cause a yellow or brown tint, while boron can result in a blue color. These are not part of the recipe but are considered impurities that alter the final appearance.
Making the Right Choice for Your Goal
Your understanding of a diamond's composition should be guided by what you want to know.
- If your primary focus is on geology: Recognize that diamonds are primordial crystals of pure carbon, formed by the mantle's immense heat and pressure, not from coal.
- If your primary focus is on materials science: Understand that both natural and lab-grown diamonds are chemically identical allotropes of carbon, distinguished only by their origin story.
- If your primary focus is on value: Appreciate that purity is key, and while the core ingredient is just carbon, trace impurities of other elements are what create rare and valuable colored diamonds.
Ultimately, the story of the diamond is a lesson in how extraordinary conditions can transform the ordinary into something exceptional.
Summary Table:
| Component | Role in Diamond Formation |
|---|---|
| Carbon | The single essential element; the building block of the diamond's atomic structure. |
| High Pressure | Forces carbon atoms into a tight, stable crystal lattice (over 725,000 psi). |
| High Temperature | Provides the energy needed for atomic rearrangement (over 2,000°F / 1,100°C). |
Ready to explore the science of materials transformation in your own lab? KINTEK specializes in high-performance lab equipment, including systems that replicate the extreme conditions needed for advanced material synthesis. Whether you're researching crystal growth, material properties, or high-pressure processes, our expertise and reliable equipment can help you achieve exceptional results. Contact our experts today to discuss how we can support your laboratory's unique needs with precision-engineered solutions.
Visual Guide
Related Products
- Manual High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
- Automatic High Temperature Heated Hydraulic Press Machine with Heated Plates for Lab
- Vacuum Hot Press Furnace Machine Heated Vacuum Press
- Warm Isostatic Press WIP Workstation 300Mpa for High Pressure Applications
- 24T 30T 60T Heated Hydraulic Press Machine with Heated Plates for Laboratory Hot Press
People Also Ask
- What is the function of a laboratory high-temperature hydraulic press? Optimize MEA Fabrication for HCl Electrolysis
- What is a hot hydraulic press? Harness Heat and Pressure for Advanced Manufacturing
- How much force can a hydraulic press exert? Understanding its immense power and design limits.
- How much psi can a hydraulic press make? From 2,000 PSI to over 50,000 PSI Explained
- What is a heated hydraulic press used for? Essential Tool for Curing, Molding, and Laminating



















