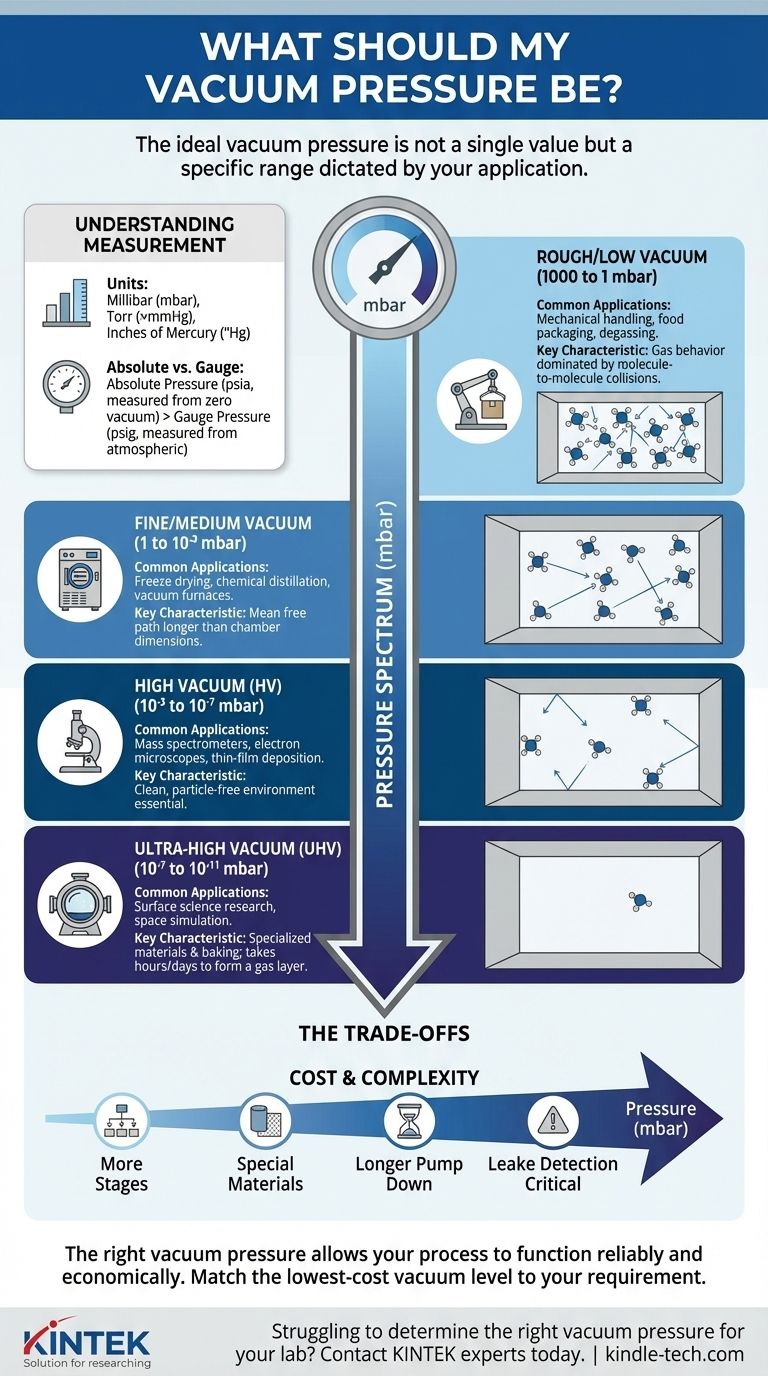
The ideal vacuum pressure is not a single value but a specific range dictated entirely by your application. While atmospheric pressure at sea level is approximately 1000 millibar (mbar), a "rough" industrial vacuum for holding parts might be 800-900 mbar, whereas a scientific instrument like a particle accelerator requires an "ultra-high" vacuum a trillion times lower.
The central challenge is not to achieve the lowest possible pressure, but to identify the appropriate level of vacuum for your specific process. Choosing a vacuum level that is too high is inefficient and costly, while choosing one that is too low will cause your process to fail.

Understanding Vacuum Measurement
To determine your target pressure, you must first understand what you are measuring. A vacuum is a space with a gaseous pressure significantly lower than the surrounding atmospheric pressure.
What Pressure Units Mean
Pressure is the force exerted by gas molecules colliding with the surfaces of a container. A "perfect" vacuum has zero pressure and no molecules.
We measure this pressure in several units. The most common are:
- Millibar (mbar): A standard metric unit. Sea level atmospheric pressure is approximately 1013 mbar.
- Torr: Nearly identical to millimeters of mercury (mmHg). One atmosphere is 760 Torr.
- Inches of Mercury ("Hg): Often used for rough vacuum. Standard atmospheric pressure is 29.92 "Hg.
Absolute vs. Gauge Pressure
It's critical to distinguish between absolute pressure (psia), which is measured relative to a perfect vacuum (zero), and gauge pressure (psig), which is measured relative to the surrounding atmospheric pressure. In vacuum science, we almost always use absolute pressure.
The Spectrum of Vacuum Levels
Vacuum is not a single state but a vast spectrum. Each level enables different physical and chemical processes, and requires different equipment.
Rough/Low Vacuum (1000 to 1 mbar)
This is the most common and least expensive vacuum level to achieve. It involves removing the bulk of the air from a chamber.
- Common Applications: Mechanical handling (vacuum chucks, lifters), food packaging, degassing of liquids, vacuum filtration.
- Key Characteristic: At this level, gas behavior is dominated by molecule-to-molecule collisions.
Fine/Medium Vacuum (1 to 10⁻³ mbar)
This range moves beyond simple air removal and begins to significantly alter the properties of materials.
- Common Applications: Freeze drying, chemical distillation, vacuum furnaces, decorative coating.
- Key Characteristic: The distance molecules travel before hitting each other (the "mean free path") becomes longer than the chamber dimensions.
High Vacuum (HV) (10⁻³ to 10⁻⁷ mbar)
At this level, the number of gas molecules is so low that they rarely collide with each other, mostly interacting with the chamber walls. This is the realm of sensitive analytical instruments.
- Common Applications: Mass spectrometers, electron microscopes, thin-film deposition (PVD), particle accelerators.
- Key Characteristic: A clean, particle-free environment is essential for processes involving electron or ion beams.
Ultra-High Vacuum (UHV) (10⁻⁷ to 10⁻¹¹ mbar)
UHV creates an almost perfect, atomically clean surface environment. Achieving and maintaining this level requires specialized materials, pumps, and baking procedures to drive off adsorbed gases.
- Common Applications: Fundamental surface science research, particle physics experiments, space simulation chambers.
- Key Characteristic: The time it takes for a single layer of gas molecules to form on a clean surface can be extended from seconds (in HV) to hours or days.
Understanding the Trade-offs
Pursuing a lower pressure (a "deeper" vacuum) is not always better. The cost and complexity increase exponentially as you move down the pressure spectrum.
Equipment Complexity and Cost
Achieving a rough vacuum may only require a single, inexpensive rotary vane pump. Reaching high vacuum requires a multi-stage system, such as a roughing pump paired with a turbomolecular or diffusion pump, which is significantly more expensive and complex to operate.
Time and Materials
Pumping down to high or ultra-high vacuum can take hours or even days. The materials used for the chamber and components become critical, as outgassing—the release of trapped gases from the material itself—becomes the primary source of gas and limits the ultimate pressure.
Leak Detection
In a rough vacuum system, a small leak may be insignificant. In a UHV system, a microscopic leak that is undetectable by normal means can prevent the system from ever reaching its target pressure, requiring sophisticated helium leak detectors to find.
Making the Right Choice for Your Goal
To determine your ideal vacuum pressure, match the lowest-cost vacuum level to your process requirement.
- If your primary focus is mechanical work or bulk processing: A rough vacuum (1 to 900 mbar) is almost always sufficient, cost-effective, and fast to achieve.
- If your primary focus is industrial drying, distillation, or metallurgy: A medium vacuum (1 to 10⁻³ mbar) is your target range.
- If your primary focus is analytical science, surface coating, or beam-line physics: You must operate in the high vacuum (HV) range (10⁻³ to 10⁻⁷ mbar).
- If your primary focus is fundamental surface research or simulating outer space: Ultra-high vacuum (UHV) is non-negotiable and requires specialized system design and expertise.
Ultimately, the right vacuum pressure is the one that allows your process to function reliably and economically.
Summary Table:
| Vacuum Level | Pressure Range (mbar) | Common Applications | Key Characteristics |
|---|---|---|---|
| Rough/Low Vacuum | 1000 to 1 | Mechanical handling, food packaging, degassing | Dominated by molecule-to-molecule collisions |
| Fine/Medium Vacuum | 1 to 10⁻³ | Freeze drying, chemical distillation, vacuum furnaces | Mean free path longer than chamber dimensions |
| High Vacuum (HV) | 10⁻³ to 10⁻⁷ | Mass spectrometers, electron microscopes, thin-film deposition | Clean, particle-free environment essential |
| Ultra-High Vacuum (UHV) | 10⁻⁷ to 10⁻¹¹ | Surface science research, space simulation | Requires specialized materials and baking procedures |
Struggling to determine the right vacuum pressure for your lab equipment? KINTEK specializes in providing precise vacuum solutions tailored to your specific laboratory needs. Whether you require a simple rough vacuum system or a complex ultra-high vacuum setup, our expertise ensures optimal performance and cost-efficiency. Let us help you achieve reliable and economical results. Contact our experts today to discuss your application and get a customized solution!
Visual Guide
Related Products
- Circulating Water Vacuum Pump for Laboratory and Industrial Use
- Oil Free Diaphragm Vacuum Pump for Laboratory and Industrial Use
- Laboratory Rotary Vane Vacuum Pump for Lab Use
- 304 316 Stainless Steel Vacuum Ball Valve Stop Valve for High Vacuum Systems
- Vacuum Hot Press Furnace Machine Heated Vacuum Press
People Also Ask
- How does the impeller rotation affect the gas flow in a water circulating vacuum pump? A Guide to the Liquid Ring Principle
- How does a water circulating vacuum pump operate? Discover the Efficient Liquid Piston Principle
- What types of gases can a water circulating vacuum pump handle? Safely Manage Flammable, Condensable & Dirty Gases
- What determines the vacuum degree achievable by a water circulating vacuum pump? Unlock the Physics of Its Limits
- What can I use a vacuum pump for? Powering Industrial Processes from Packaging to Automation



















