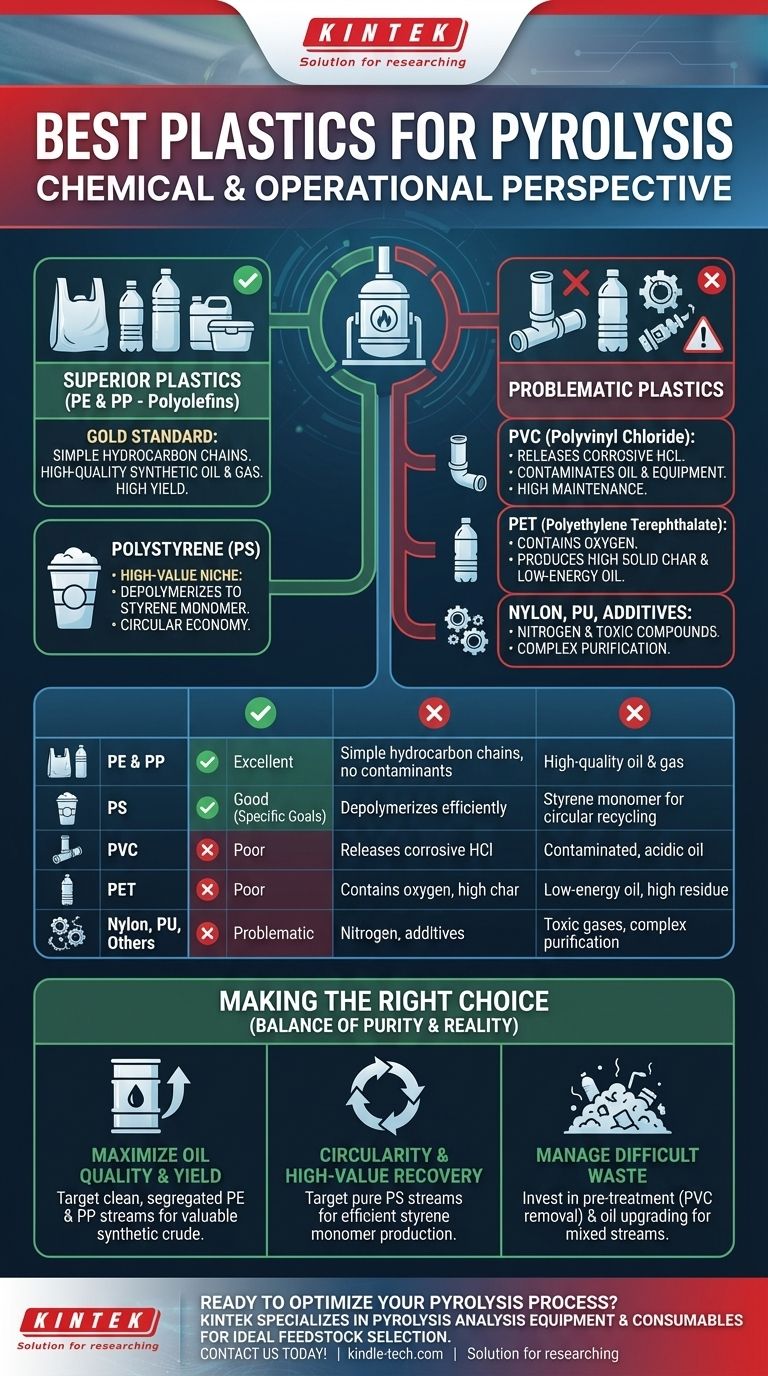
From a chemical and operational standpoint, the best plastics for pyrolysis are लाभप्रद रूप से Polyethylene (PE) and Polypropylene (PP). These plastics, known as polyolefins, consist of simple hydrocarbon chains that break down cleanly into high-quality synthetic oil and gas. While other plastics can be processed, they introduce contaminants and operational challenges that significantly reduce the efficiency and value of the final products.
The ideal plastic for pyrolysis is not just a matter of polymer type, but a balance between chemical suitability and feedstock purity. While PE and PP offer the best chemical foundation, the 'best' practical option is often a clean, well-sorted waste stream that minimizes operational costs and maximizes output value.

Why Some Plastics are Superior for Pyrolysis
Pyrolysis is a process of thermal decomposition in the absence of oxygen. The goal is typically to break down long polymer chains into smaller, more valuable liquid hydrocarbon molecules (pyrolysis oil). The chemical structure of the input plastic directly determines the quality and composition of this output.
The Gold Standard: Polyolefins (PE & PP)
Polyethylene (PE)—found in plastic bags, bottles, and films—and Polypropylene (PP)—used in containers, car parts, and fibers—are the most desirable feedstocks.
Their structure consists solely of carbon and hydrogen. When heated, they break down into a mix of paraffins and olefins, which are chemically similar to a crude oil fraction. This results in a high-yield, high-quality pyrolysis oil with good energy content and fewer impurities.
A High-Value Niche: Polystyrene (PS)
Polystyrene (PS), used in foam cups and packaging, is also a strong candidate, but for a different reason.
Under the right pyrolysis conditions, PS can "unzip" or depolymerize back into its original building block: styrene monomer. This liquid can be purified and used to make new polystyrene, representing a true circular economy pathway. The yield of styrene monomer can be very high, making it economically attractive if a pure PS waste stream is available.
Problematic Plastics and Their Challenges
Not all plastics are created equal. Certain polymers introduce contaminants that can corrode equipment, poison catalysts, and create a low-value, toxic output. Understanding these limitations is critical for any viable pyrolysis operation.
The PVC Problem: Chlorine Contamination
Polyvinyl Chloride (PVC) is the most problematic contaminant in a pyrolysis feedstock.
When heated, PVC releases a significant amount of hydrochloric acid (HCl). This acid is highly corrosive to reactors and piping, leading to frequent and expensive maintenance. It also contaminates the pyrolysis oil, making it acidic and unusable without costly and complex purification steps. Even small amounts of PVC can compromise an entire batch.
The PET Challenge: Oxygen and Solid Residue
Polyethylene Terephthalate (PET), the plastic used in water and soda bottles, is also a suboptimal feedstock.
PET's chemical structure contains oxygen, which ends up in the pyrolysis oil and water, reducing the oil's energy content and stability. Furthermore, PET tends to produce a higher amount of solid char compared to PE or PP, which lowers the overall liquid yield and complicates reactor operation.
Other Contaminants: Nitrogen and Additives
Plastics like Nylon (Polyamide) and Polyurethane (PU) contain nitrogen. During pyrolysis, this can form nitrogen-containing compounds in the oil or release potentially toxic gases. Similarly, additives like flame retardants, plasticizers, and pigments can end up in the oil or char, complicating its downstream use.
Understanding the Trade-offs: Purity vs. Reality
While pure PE and PP are chemically ideal, they are rarely found in isolation. The real-world challenge of pyrolysis is managing mixed and contaminated waste streams.
Feedstock Purity is Paramount
The single greatest factor determining the success of a pyrolysis plant is the quality of its input. Cleaner, better-sorted feedstock leads to higher-quality oil, lower operational costs, and a more stable process. Investment in pre-sorting and cleaning technology is not an option; it is a necessity.
The Reality of Mixed Waste Streams
Pyrolysis is often proposed as a solution for waste that cannot be mechanically recycled, such as multi-layer packaging or municipal solid waste. While it can process these streams, it comes at a cost.
A system designed for mixed waste must have robust pre-treatment to remove PVC and other contaminants. It must also include extensive post-treatment systems to upgrade the contaminated, low-quality oil into a usable product. This significantly increases both capital and operational expenses.
Making the Right Choice for Your Goal
The "best" plastic feedstock is directly tied to your primary objective.
- If your primary focus is maximizing oil quality and yield: Target clean, segregated streams of Polyethylene (PE) and Polypropylene (PP) to produce a valuable synthetic crude oil substitute.
- If your primary focus is circularity and high-value chemical recovery: Target pure streams of Polystyrene (PS) to efficiently produce styrene monomer for new polymer production.
- If your primary focus is managing difficult-to-recycle waste: Prepare to invest heavily in pre-treatment (especially PVC removal) and oil upgrading technology to handle the operational realities of mixed plastic feedstock.
Ultimately, selecting the right plastic feedstock is a strategic decision that balances chemical ideals with the economic and logistical realities of waste management.
Summary Table:
| Plastic Type | Suitability for Pyrolysis | Key Characteristics | Primary Output |
|---|---|---|---|
| Polyethylene (PE) & Polypropylene (PP) | Excellent | Simple hydrocarbon chains, no contaminants | High-quality synthetic oil & gas |
| Polystyrene (PS) | Good (for specific goals) | Depolymerizes efficiently | Styrene monomer for circular recycling |
| PVC (Polyvinyl Chloride) | Poor | Releases corrosive hydrochloric acid (HCl) | Contaminated, acidic oil |
| PET (Polyethylene Terephthalate) | Poor | Contains oxygen, produces solid char | Low-energy oil, high residue |
| Nylon, Polyurethane | Problematic | Contains nitrogen, additives | Toxic gases, complex purification needed |
Ready to optimize your pyrolysis process with the right feedstock? KINTEK specializes in lab equipment and consumables for pyrolysis analysis, helping you test and select the ideal plastics for maximum efficiency and value. Whether you're targeting high-yield oil from PE/PP or circular recovery from PS, our solutions ensure accurate, reliable results. Contact us today to discuss your laboratory needs and discover how KINTEK can support your pyrolysis projects!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
People Also Ask
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process



















