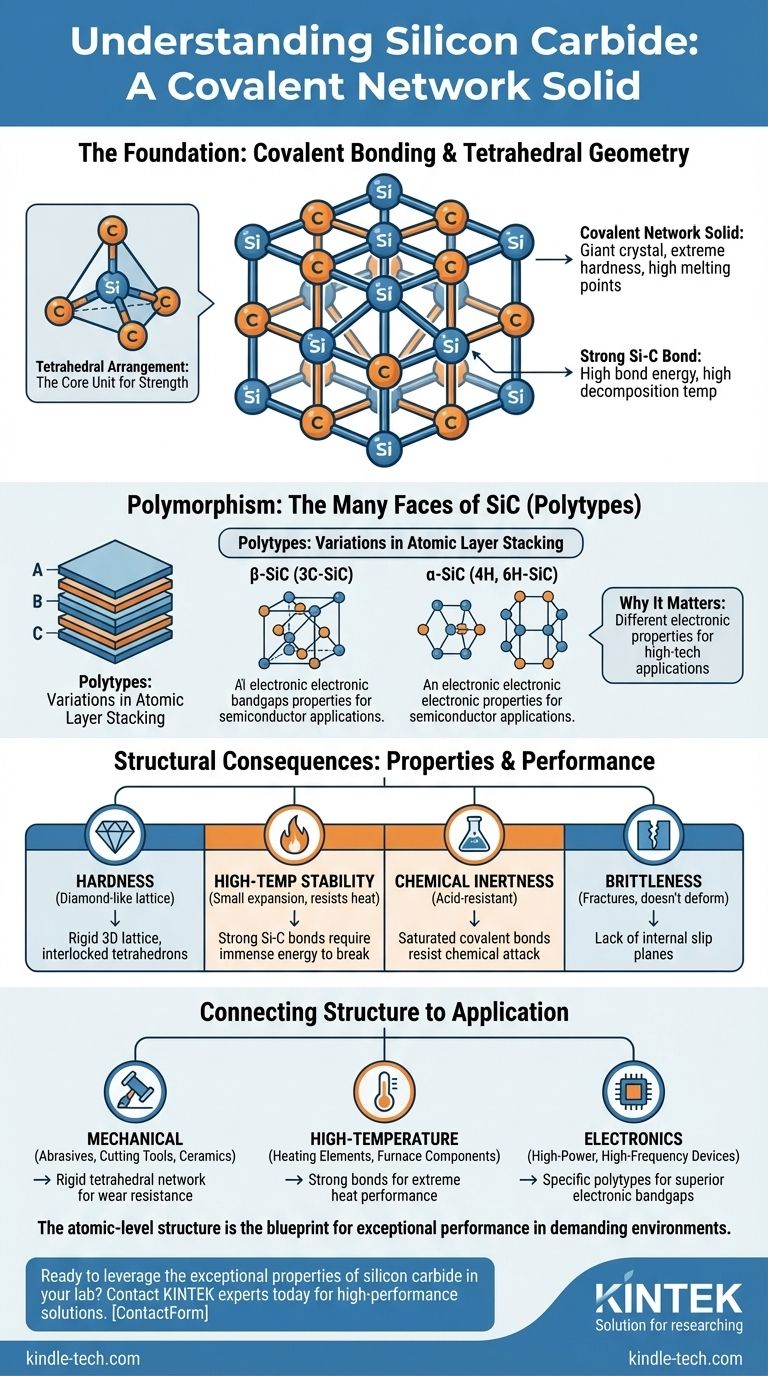
At its core, silicon carbide (SiC) is a covalent network solid. This means its fundamental structure is a vast, three-dimensional crystal lattice built from silicon (Si) and carbon (C) atoms. Each atom is tightly locked in place by strong covalent bonds in a repeating tetrahedral pattern, much like the structure of diamond. This arrangement can form in many different stacking variations, known as polytypes.
The question of silicon carbide's structure is central to understanding its remarkable properties. Its rigid, diamond-like covalent network is the direct cause of its exceptional hardness, chemical stability, and ability to withstand extreme temperatures.

The Foundation: Covalent Bonding and Tetrahedral Geometry
Understanding SiC begins with its atomic arrangement. Unlike metals with free-floating electrons or salts held by ionic attraction, SiC's strength comes from strong, shared electron bonds that create a single, massive molecule.
What is a Covalent Network Solid?
A covalent network solid is a substance where atoms are bonded by covalent bonds in a continuous network extending throughout the material. There are no individual molecules.
The entire crystal is essentially one giant molecule. This structure is responsible for the extreme hardness and high melting points of materials like diamond and silicon carbide.
The Tetrahedral Arrangement
In the SiC lattice, every silicon atom is chemically bonded to four neighboring carbon atoms. Likewise, every carbon atom is bonded to four neighboring silicon atoms.
This arrangement forms a tetrahedron, a highly stable and symmetrical geometric shape. This repeating pattern of interlocked tetrahedrons creates an incredibly strong and rigid framework.
The Strength of the Si-C Bond
The bond between silicon and carbon is very strong and relatively short. A significant amount of energy is required to break it.
This high bond energy is the direct source of SiC's high decomposition temperature (it sublimes rather than melts) and its exceptional hardness, which is surpassed by very few materials.
Polymorphism: The Many Faces of Silicon Carbide
Silicon carbide is not just one single structure. It exhibits a phenomenon called polymorphism, meaning it can exist in many different crystal structures while maintaining the same chemical formula (SiC). These different forms are called polytypes.
Understanding Polytypes
Polytypes are variations in the stacking sequence of the atomic layers in the crystal. Imagine stacking layers of atoms represented by A, B, and C. Different repeating patterns (like ABCABC... or ABAB...) result in different crystal structures.
While chemically identical, these polytypes can have distinct physical and, most importantly, electronic properties.
Key Categories: α-SiC and β-SiC
The hundreds of known SiC polytypes are broadly grouped into two main categories.
Beta-SiC (β-SiC) refers to the cubic polytype (3C-SiC), which has a structure similar to zincblende. Alpha-SiC (α-SiC) encompasses all other polytypes, which are primarily hexagonal (like 4H-SiC and 6H-SiC) or rhombohedral.
Why Polytypes Matter for Applications
The existence of polytypes is not just an academic detail; it's critical for high-tech applications. Different polytypes have different electronic bandgaps, which determines their semiconductor properties.
For example, the 4H-SiC polytype is favored for high-power, high-frequency electronic devices because its electronic properties are superior for that specific purpose, underpinning its use in the advanced semiconductor field.
Understanding the Structural Consequences
The properties listed for silicon carbide—hardness, stability, and temperature resistance—are all direct consequences of its underlying atomic structure.
Extreme Hardness, but High Brittleness
The reference notes that SiC is "hard and brittle." The rigid covalent lattice that provides its extreme hardness also means it lacks the internal slip planes found in metals.
When subjected to stress beyond what the bonds can withstand, the crystal cannot deform by allowing atoms to slide past one another. Instead, it fractures catastrophically, which is the definition of brittleness.
High-Temperature Stability
The powerful Si-C bonds require immense thermal energy to vibrate and break apart. This is why SiC is described as having a "small expansion coefficient" and "good resistance to rapid cooling and heat."
This thermal stability makes it an ideal material for high-temperature electric heating elements and furnace components, as it maintains its structural integrity at temperatures where most metals would melt or deform.
Chemical Inertness
The reference highlights SiC's "good chemical stability" and notes it is "extremely acid-resistant." The stable, saturated covalent bonds are not easily attacked or broken by chemical reagents.
The electrons are locked tightly between the silicon and carbon atoms, leaving few opportunities for acids or other chemicals to react, resulting in a highly durable and non-reactive material.
Connecting Structure to Application
Understanding silicon carbide's atomic structure allows you to select it for the right purpose with confidence. The properties are not arbitrary; they are a direct result of its covalent network.
- If your primary focus is mechanical strength and wear resistance: The rigid, interconnected tetrahedral network makes SiC an ideal choice for abrasives, cutting tools, and durable structural ceramics.
- If your primary focus is high-temperature performance: The high energy required to break its Si-C bonds makes it perfect for heating elements, furnace components, and refractory materials.
- If your primary focus is advanced electronics: The distinct electronic properties of specific polytypes, like 4H-SiC, are critical for creating the next generation of high-power and high-frequency semiconductor devices.
Ultimately, the atomic-level structure of silicon carbide is the direct blueprint for its exceptional performance in the world's most demanding environments.
Summary Table:
| Property | Consequence of Structure |
|---|---|
| Hardness | Rigid, 3D covalent lattice of interlocked tetrahedrons |
| High-Temp Stability | Strong Si-C bonds require immense energy to break |
| Chemical Inertness | Saturated covalent bonds resist chemical attack |
| Brittleness | Lack of slip planes in the rigid lattice leads to fracture |
| Polytypes (e.g., 4H-SiC) | Different atomic layer stacking sequences enable advanced semiconductor applications |
Ready to leverage the exceptional properties of silicon carbide in your lab?
At KINTEK, we specialize in high-performance lab equipment and consumables built to thrive in demanding environments. Whether your application requires extreme wear resistance, high-temperature stability, or advanced semiconductor capabilities, our expertise in materials science can help you achieve superior results.
Contact our experts today to discuss how our silicon carbide solutions can enhance your laboratory's efficiency and capabilities.
Visual Guide
Related Products
- Conductive Boron Nitride BN Ceramics Composite for Advanced Applications
- Conductive Carbon Cloth Carbon Paper Carbon Felt for Electrodes and Batteries
- Precision Machined Zirconia Ceramic Ball for Engineering Advanced Fine Ceramics
- Laboratory CVD Boron Doped Diamond Materials
- Laboratory Vibratory Sieve Shaker Machine for Dry and Wet Three-Dimensional Sieving
People Also Ask
- What is the maximum temperature for ceramics? Find the Right Material for Your High-Temp Application
- What are the strengths of brazing? Achieve Strong, Clean, and Precise Metal Joining
- What are the classification of ceramic materials? A Guide to Oxides, Non-Oxides, and Composites
- How long does it take to solder? A guide to timing and technique for perfect joints
- Why is a hexagonal Boron Nitride (h-BN) layer required for LATP? Protect Your Samples from Carbon Contamination



















