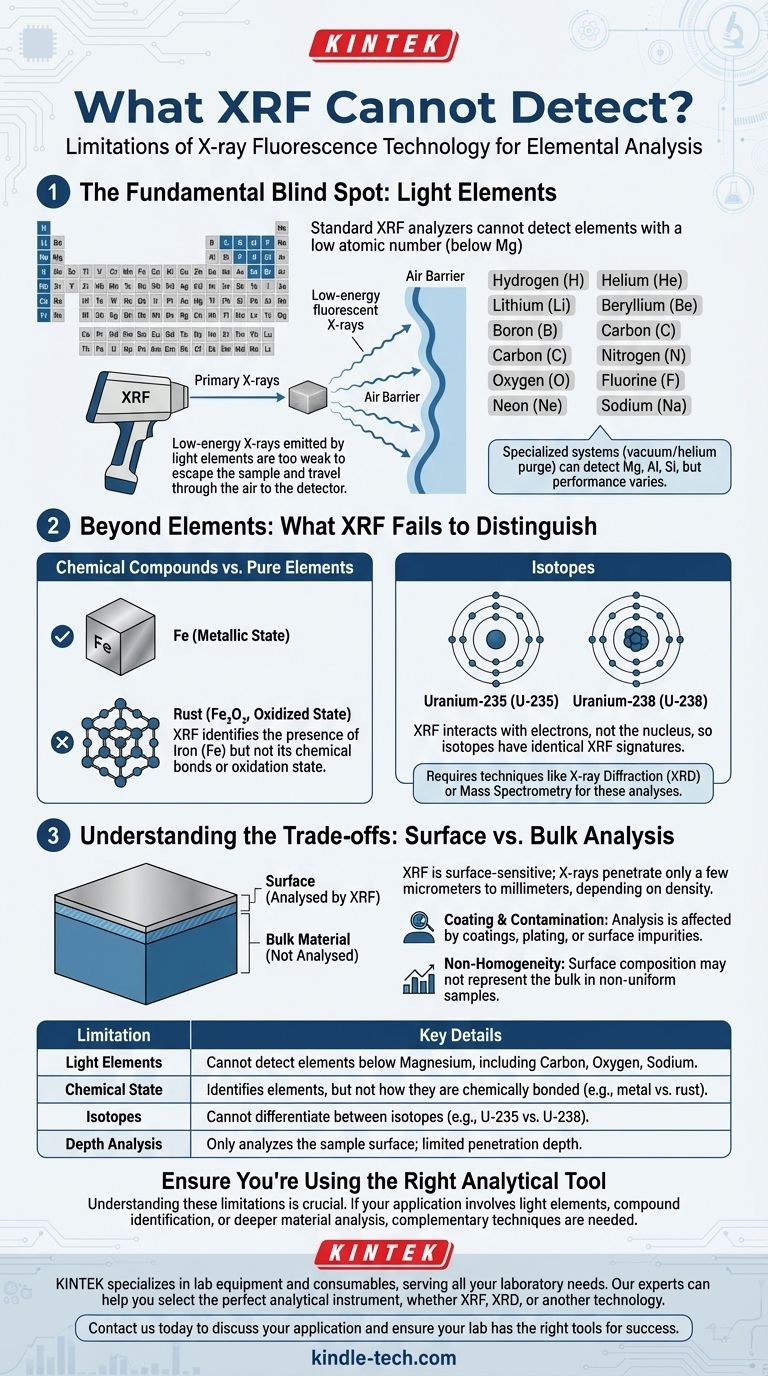
At its core, X-ray Fluorescence (XRF) is a powerful and rapid technology for elemental analysis, but it has distinct and fundamental limitations. Primarily, standard XRF analyzers cannot detect very light elements, are unable to identify the specific chemical compounds an element has formed, and can only analyze the surface of a sample.
The crucial takeaway is that XRF identifies which elements are present and in what quantity, but not how they are chemically bonded or what lies beneath the immediate surface. Its most significant blind spot is for elements with a low atomic number, such as carbon, oxygen, and sodium.

The Fundamental Blind Spot: Light Elements
The most well-known limitation of XRF is its inability to detect elements at the top of the periodic table. This is not a design flaw but a consequence of the physics involved.
Why Atomic Number Matters
XRF works by measuring the energy of fluorescent X-rays emitted from a sample. Lighter elements, those with a low atomic number (generally below magnesium, Mg), emit very low-energy X-rays.
These low-energy X-rays are not powerful enough to escape the sample itself, pass through the air, and reach the instrument's detector in sufficient numbers to be measured reliably.
The "Air Barrier"
The air between the sample and the XRF detector is a major obstacle for low-energy X-rays. Nitrogen and oxygen molecules in the air readily absorb them, preventing a measurement.
Specialized laboratory systems can overcome this by creating a vacuum or purging the chamber with helium, but this is not a feature of standard handheld units.
Which Elements Are Typically Invisible?
For most portable XRF analyzers, the list of undetectable elements includes the first 11 on the periodic table: Hydrogen (H), Helium (He), Lithium (Li), Beryllium (Be), Boron (B), Carbon (C), Nitrogen (N), Oxygen (O), Fluorine (F), Neon (Ne), and Sodium (Na). Some high-end models can detect Magnesium (Mg), Aluminum (Al), and Silicon (Si), but performance varies.
Beyond Elements: What XRF Fails to Distinguish
An element's presence is only part of the story. XRF cannot provide information about chemical structure or isotopic composition.
Chemical Compounds vs. Pure Elements
XRF tells you that iron (Fe) is present, but it cannot tell you if that iron is in a metallic state (like in stainless steel) or an oxidized state (like rust, Fe₂O₃). The analysis is purely elemental.
To determine the specific compound or mineral phase, you would need a different technique like X-ray Diffraction (XRD).
The Inability to Distinguish Isotopes
The XRF process interacts with the electron shells of an atom, not its nucleus. Since isotopes of an element have the same number of electrons, their XRF signatures are identical.
Therefore, XRF cannot distinguish between Uranium-235 and Uranium-238, or any other isotopes. This requires mass spectrometry.
Understanding the Trade-offs: Surface vs. Bulk Analysis
A common misconception is that XRF provides a complete analysis of an entire object. In reality, it is a surface-sensitive technique.
The Limitation of Penetration Depth
The X-rays from the analyzer only penetrate a very shallow depth into the sample, typically from a few micrometers to several millimeters. The exact depth depends on the material's density and the energy of the X-rays.
This means the analysis you receive is only representative of the material at or near the surface.
The Critical Role of Sample Homogeneity
If a sample is not uniform throughout (non-homogeneous), the surface analysis from an XRF will not match the bulk composition. An analysis of a rock, for example, will only reflect the mineral composition on its immediate surface.
The Problem with Coatings and Contamination
Because XRF analyzes the surface, any coating, plating, or even significant contamination will be what the instrument measures.
An XRF shot of a zinc-plated steel bolt will report high levels of zinc, potentially missing the underlying steel entirely. The surface must be clean and representative of the material you intend to measure.
Is XRF the Right Tool for Your Task?
Understanding these limitations is key to using the technology effectively. The choice depends entirely on the question you need to answer.
- If your primary focus is rapid alloy identification, RoHS compliance, or heavy metal screening in soil: XRF is an excellent, fast, and reliable choice because these applications depend on detecting medium to heavy elements.
- If your primary focus is analyzing polymers, hydrocarbons, or other organic materials: You must use an alternative method. XRF cannot detect the core C, H, and O elements that define these materials.
- If your primary focus is identifying the specific mineral, chemical compound, or isotopic ratio: XRF is not the correct tool. You need complementary techniques like XRD or mass spectrometry.
Ultimately, knowing what a tool cannot do is just as important as knowing what it can.
Summary Table:
| Limitation | Key Details |
|---|---|
| Light Elements | Cannot detect elements with low atomic numbers (typically below Magnesium), including Carbon (C), Oxygen (O), and Sodium (Na). |
| Chemical State | Identifies which elements are present but cannot determine how they are chemically bonded (e.g., cannot distinguish between metal and rust). |
| Isotopes | Cannot differentiate between isotopes of an element (e.g., U-235 vs. U-238). |
| Depth Analysis | Only analyzes the surface of a sample; penetration depth is limited. |
Ensure You're Using the Right Analytical Tool
Understanding the limitations of XRF is crucial for accurate results. If your application involves light elements, compound identification, or deeper material analysis, you may need a complementary technique.
KINTEK specializes in lab equipment and consumables, serving all your laboratory needs. Our experts can help you select the perfect analytical instrument for your specific requirements, whether it's XRF, XRD, or another technology.
Contact us today to discuss your application and ensure your lab has the right tools for success.
Visual Guide
Related Products
- Lab Electrochemical Workstation Potentiostat for Laboratory Use
- Three-dimensional electromagnetic sieving instrument
- Custom PTFE Teflon Parts Manufacturer for PTFE Tweezers
- Optical Window Glass Substrate Wafer CaF2 Substrate Window Lens
- Infrared High Resistance Single Crystal Silicon Lens
People Also Ask
- Does tensile strength increase with heat treatment? How to Engineer the Perfect Metal Properties
- How does a precision heating system influence the coating quality of soft magnetic composite materials? Expert Insights
- What is the long-term stability of viral analytes in plasma stored at -70°C? Proven for Decades of Research
- What is KBR technology? The Blueprint for Industrial-Scale Chemical Production
- What is the principle of quenching effect? Harnessing Molecular Interactions to Control Fluorescence
- What is the process of thin film in semiconductor? Build the Layers of Modern Electronics
- Why argon gas is used in sputtering? Achieve Pure, Cost-Effective Thin Film Deposition
- What is the meaning of vacuum pyrolysis? Maximize Liquid Fuel Yield from Waste













