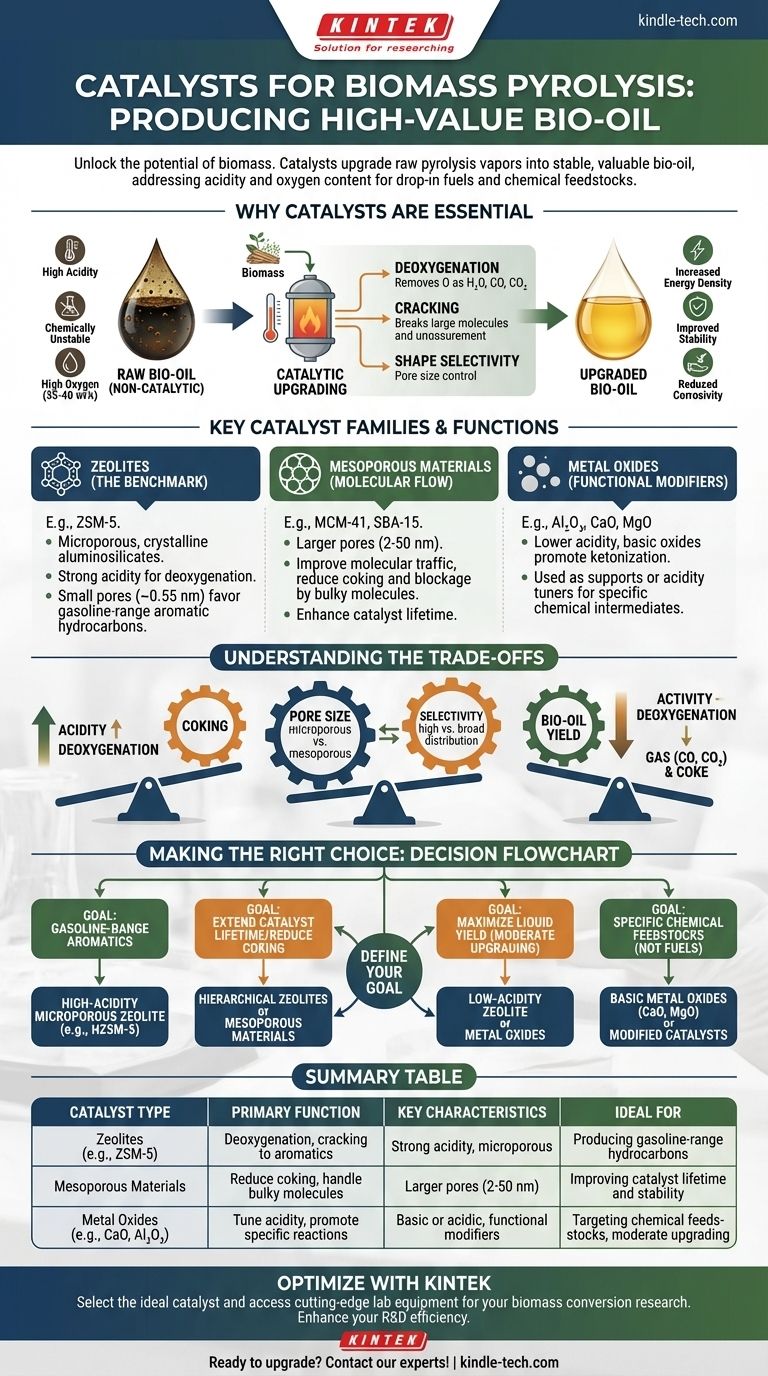
In biomass pyrolysis, the most common catalysts are microporous zeolites, particularly ZSM-5, which are used to upgrade the quality of raw pyrolysis vapors into a more stable, higher-value bio-oil. Alongside zeolites, other materials like mesoporous catalysts and various metal oxides are employed to fine-tune the process, reduce undesirable byproducts, and target specific chemical compounds.
The core challenge is not finding a single "best" catalyst, but rather selecting or designing a catalyst whose specific properties—such as acidity and pore structure—are precisely matched to the biomass feedstock and the desired characteristics of the final bio-oil.

Why Catalysts Are Essential in Pyrolysis
Raw bio-oil, produced from non-catalytic pyrolysis, is a complex mixture with significant drawbacks. It is highly acidic, chemically unstable, and contains a large amount of oxygen (35-40 wt%).
These properties make it corrosive and incompatible with existing petroleum refinery infrastructure. The primary goal of catalytic pyrolysis is to "upgrade" this oil by removing oxygen and cracking large molecules into smaller, more valuable ones.
The Role of Deoxygenation
Catalysts facilitate deoxygenation reactions, removing oxygen atoms from organic molecules in the form of water (dehydration), carbon monoxide (decarbonylation), and carbon dioxide (decarboxylation). This process is critical for increasing the energy density and stability of the bio-oil.
Cracking and Shape Selectivity
Catalysts also possess acidic sites that break down large, complex molecules from the biomass into smaller, more useful hydrocarbons. The physical structure of the catalyst, specifically its pore size, can control which molecules are formed—a principle known as shape selectivity.
Key Catalyst Families and Their Functions
The choice of catalyst directly dictates the chemical pathways available and, therefore, the composition of the final bio-oil. The main families are distinguished by their structure and chemical nature.
Zeolites: The Industry Benchmark
Zeolites are crystalline aluminosilicates with a well-defined microporous structure. ZSM-5 is the most widely studied and utilized zeolite for this application.
Its strong acidity is highly effective at deoxygenation, and its small pore size (~0.55 nm) preferentially produces gasoline-range aromatic hydrocarbons. This makes it a benchmark for producing drop-in fuels.
Mesoporous Materials: Improving Molecular Flow
While effective, the small pores of conventional zeolites can become easily blocked by the bulky molecules derived from lignin and cellulose. This leads to rapid deactivation by coke formation.
Mesoporous materials, such as MCM-41 and SBA-15, feature larger pores (2-50 nm). These materials improve "molecular traffic control," allowing larger molecules to enter and react, which can reduce coking and improve catalyst lifetime. Often, they are used in hierarchical structures that combine micro- and mesopores.
Metal Oxides: The Functional Modifiers
Simple metal oxides like Al₂O₃ (alumina), CaO (calcium oxide), and MgO (magnesium oxide) are also used. They typically have lower acidity than zeolites.
Basic oxides (CaO, MgO) can promote different reactions, such as ketonization, which can be valuable for producing specific chemical intermediates rather than fuel-range hydrocarbons. They can also be used as catalyst supports or as additives to tune the acidity of a primary catalyst like a zeolite.
Understanding the Trade-offs
There is no perfect catalyst. Selecting one involves navigating a series of critical trade-offs that impact efficiency, cost, and the final product.
Acidity vs. Coking
Strong acid sites are excellent for deoxygenation but also accelerate coke formation. Coke is a carbonaceous deposit that covers the active sites of the catalyst, rendering it inactive. This creates a constant operational challenge of balancing high activity with catalyst stability and regeneration frequency.
Pore Size vs. Selectivity
Microporous zeolites like ZSM-5 offer exceptional shape selectivity for producing valuable aromatics. However, their small pores are prone to clogging. Mesoporous catalysts solve the clogging issue but offer less control over the final product distribution, often leading to a wider range of less-specific molecules.
Activity vs. Bio-oil Yield
Aggressive catalytic upgrading that maximizes deoxygenation and aromatic production often comes at the cost of overall liquid bio-oil yield. A significant portion of the biomass carbon is lost to the gas phase (CO, CO₂) and solid coke. The most active catalyst does not always produce the most liquid fuel.
Making the Right Choice for Your Goal
The optimal catalyst is entirely dependent on your primary objective. Before selection, clearly define what success looks like for your process.
- If your primary focus is producing gasoline-range aromatic hydrocarbons: A high-acidity, microporous zeolite like HZSM-5 is the established industry standard and your best starting point.
- If your primary focus is extending catalyst lifetime and reducing coking: Investigate hierarchical zeolites or mesoporous materials that improve access for bulky molecules and reduce pore blockage.
- If your primary focus is maximizing the liquid yield with moderate upgrading: A less acidic catalyst, such as a low-acidity zeolite or certain metal oxides, may be preferable to minimize gas and coke formation.
- If your primary focus is producing specific chemical feedstocks (not fuels): Explore basic metal oxides (CaO, MgO) or modified catalysts designed to promote alternative reaction pathways like ketonization or aldol condensation.
Ultimately, effective biomass conversion is achieved by intelligently tuning your catalyst system to meet a specific end-product goal.
Summary Table:
| Catalyst Type | Primary Function | Key Characteristics | Ideal For |
|---|---|---|---|
| Zeolites (e.g., ZSM-5) | Deoxygenation, cracking to aromatics | Strong acidity, microporous structure | Producing gasoline-range hydrocarbons |
| Mesoporous Materials (e.g., MCM-41) | Reduce coking, handle bulky molecules | Larger pores (2-50 nm) | Improving catalyst lifetime and stability |
| Metal Oxides (e.g., CaO, Al₂O₃) | Tune acidity, promote specific reactions | Basic or acidic sites, functional modifiers | Targeting chemical feedstocks, moderate upgrading |
Optimize Your Biomass Pyrolysis Process with KINTEK
Choosing the right catalyst is critical for achieving high-quality bio-oil with maximum efficiency. At KINTEK, we specialize in providing advanced laboratory equipment and consumables tailored to your biomass conversion needs. Whether you're researching zeolite performance, testing mesoporous materials, or scaling up your pyrolysis process, our solutions ensure precision and reliability.
Let us help you:
- Select the ideal catalyst for your specific biomass feedstock and desired bio-oil properties.
- Access cutting-edge lab equipment for catalyst testing, pyrolysis, and analysis.
- Enhance your R&D efficiency with our expert support and high-quality consumables.
Ready to upgrade your bio-oil production? Contact our experts today to discuss how KINTEK can support your laboratory and pyrolysis goals!
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for PTFE Measuring Cylinder 10/50/100ml
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Bomb Type Probe for Steelmaking Production Process
People Also Ask
- What are the specific applications of PTFE in micro-batch slug flow systems? Enhance Your Microfluidic Reaction Purity
- Why must a Polytetrafluoroethylene (PTFE) reactor be used for Ti3C2TX MXene etching? Ensure Safety and Purity
- What are the four main types of sensors? A Guide to Power Source and Signal Type
- What is the function of PTFE reaction kettle bodies in micro-CSTR systems? Enhance Chemical Stability & Flow
- What role do PTFE containers play in trace metal detection? Ensure Analytical Precision in Photocatalysis Studies



















