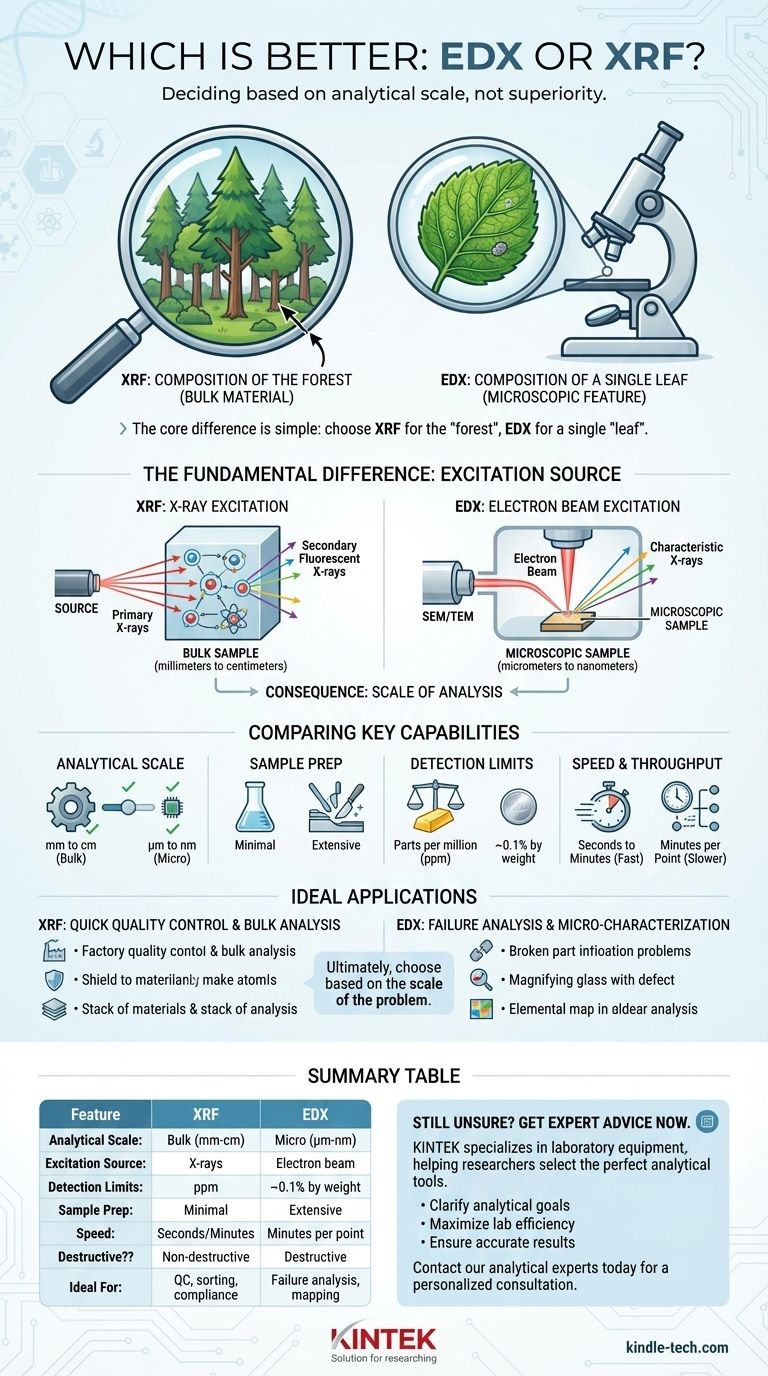
Deciding between EDX and XRF is a matter of understanding the scale of your analytical question, not determining which technology is inherently superior. The right choice depends entirely on the size of the feature you need to analyze. X-ray Fluorescence (XRF) is a bulk analysis technique ideal for determining the average elemental composition of a large sample area, while Energy Dispersive X-ray Spectroscopy (EDX) is a microanalysis technique used within an electron microscope to identify the elements in a microscopic region.
The core difference is simple: choose XRF when you need to know the composition of the "forest" (the bulk material) and choose EDX when you need to know the composition of a single "leaf" (a microscopic particle, defect, or feature).

The Fundamental Difference: How the Sample is Excited
The distinct capabilities of EDX and XRF stem directly from their source of excitation. One uses X-rays to generate X-rays, and the other uses electrons. This single difference dictates the scale, sensitivity, and application of each technique.
How XRF Works: X-ray Excitation
In XRF, a primary beam of high-energy X-rays is directed at the sample. This beam is powerful enough to penetrate the material's surface, interacting with a relatively large volume of atoms.
This interaction ejects inner-shell electrons, causing the atoms to emit secondary, "fluorescent" X-rays. The instrument measures the energy of these secondary X-rays to identify the elements present.
How EDX Works: Electron Beam Excitation
EDX (also called EDS) operates as an accessory to a Scanning Electron Microscope (SEM) or Transmission Electron Microscope (TEM). It uses a highly focused electron beam as its excitation source.
Because electrons interact very strongly with matter, they only penetrate a few micrometers into the sample's surface. This small interaction volume allows for extremely precise, high-magnification elemental analysis of specific features.
The Consequence: Scale of Analysis
The excitation source directly defines the analytical volume. XRF's penetrating X-rays analyze a spot size measured in millimeters or centimeters, providing an average composition of a bulk material.
EDX's focused electron beam analyzes a spot size measured in micrometers or even nanometers, providing the elemental makeup of a single grain, inclusion, or surface defect.
Comparing Key Analytical Capabilities
Beyond the scale of analysis, the two techniques differ in sample requirements, sensitivity, and speed.
Sample Size and Preparation
XRF is highly flexible and excels with large, bulk samples, including solids, powders, and liquids. Sample preparation is often minimal or non-existent, a key advantage for rapid screening.
EDX requires small samples that can fit inside the vacuum chamber of an electron microscope. These samples must be conductive and stable under an electron beam, often requiring meticulous preparation like cutting, polishing, and carbon coating.
Detection Limits and Sensitivity
XRF generally offers lower detection limits, capable of measuring trace elements down to parts per million (ppm) levels in many materials. It is the preferred tool for verifying compliance with standards like RoHS.
EDX is less sensitive, with typical detection limits around 0.1% by weight. The electron beam generates significant background radiation (Bremsstrahlung), which makes detecting trace elements difficult.
Element Range
Both techniques can detect elements from Sodium (Na) to Uranium (U) with standard detectors.
Specialized detectors exist for both, but EDX in particular struggles with very light elements (below Sodium, like Carbon, Oxygen, or Nitrogen) due to low X-ray yield and the physics of detection.
Understanding the Trade-offs and Practical Considerations
Choosing the right tool involves balancing analytical needs with practical constraints like cost, speed, and sample integrity.
Speed and Throughput
XRF is exceptionally fast. Handheld and benchtop analyzers can deliver a comprehensive elemental composition in seconds to a few minutes, making it ideal for high-throughput quality control and material sorting.
EDX is a much slower process. It requires loading the sample into a vacuum chamber, navigating to the precise microscopic area of interest, and then acquiring the spectrum, which can take several minutes per point.
Cost and Accessibility
An XRF instrument is a self-contained unit. Handheld and benchtop models are relatively affordable, easy to train operators on, and do not require a specialized lab environment.
EDX is an accessory to an electron microscope. The combined cost of an SEM with an EDX detector is significantly higher, and its operation requires a skilled technician in a dedicated facility.
Destructive vs. Non-destructive
XRF is almost entirely non-destructive. The primary X-ray beam does not damage or alter the sample, allowing valuable objects or components to be analyzed without harm.
EDX can be destructive in two ways. First, the required sample preparation (cutting and coating) is inherently destructive. Second, the intense electron beam can damage sensitive materials like polymers, organic matter, or ceramics.
Making the Right Choice for Your Goal
The best technique is the one that aligns with the specific question you need to answer about your material.
- If your primary focus is quick quality control or bulk composition: XRF is the clear choice for its speed, ease of use, and ability to analyze unprepared samples.
- If your primary focus is failure analysis or characterizing microscopic features: EDX is the only option, providing essential elemental data for tiny defects, particles, or phases.
- If your primary focus is detecting trace contaminants in a homogeneous material: XRF is superior due to its significantly lower detection limits.
- If your primary focus is creating elemental maps to see how elements are distributed: EDX is designed for this, allowing you to visualize the spatial distribution of elements across a surface.
Ultimately, you must choose your analytical tool based on the scale of the problem you are trying to solve.
Summary Table:
| Feature | XRF (X-ray Fluorescence) | EDX (Energy Dispersive X-ray Spectroscopy) |
|---|---|---|
| Analytical Scale | Bulk analysis (mm to cm) | Microanalysis (µm to nm) |
| Excitation Source | X-rays | Electron beam (in SEM/TEM) |
| Detection Limits | Parts per million (ppm) | ~0.1% by weight |
| Sample Prep | Minimal (solids, powders, liquids) | Extensive (cutting, polishing, coating) |
| Speed | Seconds to minutes | Minutes per analysis point |
| Destructive? | Non-destructive | Destructive (sample prep & beam damage) |
| Ideal For | Quality control, material sorting, compliance testing | Failure analysis, particle identification, elemental mapping |
Still Unsure Which Technique is Right for Your Application?
KINTEK specializes in laboratory equipment and consumables, helping researchers and quality control professionals select the perfect analytical tools for their specific needs. Whether you require bulk composition analysis with XRF or microscopic feature characterization with EDX, our experts can guide you to the optimal solution.
Let us help you:
- Clarify your analytical goals and match them with the right technology
- Maximize your lab's efficiency with equipment tailored to your workflow
- Ensure accurate results with proper technique selection and setup
Contact our analytical experts today for a personalized consultation and discover how KINTEK can enhance your elemental analysis capabilities.
Visual Guide
Related Products
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Three-dimensional electromagnetic sieving instrument
- Warm Isostatic Press for Solid State Battery Research
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Platinum Auxiliary Electrode for Laboratory Use
People Also Ask
- What is the difference between a manual press and a hydraulic press? Manual vs. Automatic Control Explained
- How is a laboratory hydraulic press used in the fabrication of graphite-cement composite electrodes?
- What is the difference between fused beads and pressed pellets? Choose the Right XRF Sample Prep Method
- What are the safety concerns of a hydraulic press? Mitigating High-Pressure and Mechanical Risks
- How strong can a hydraulic press be? From 10 to 80,000 Tons of Force Explained
- What are three ways to reduce production time in compression molding? Optimize Design, Preheat, and Automate
- What is the role of a laboratory hydraulic press in Al-doped LLZO pre-treatment? Ensure Dense Electrolyte Formation.
- What is the core function of a laboratory hydraulic press in the production of fuel cell bipolar plates? Expert Guide


















