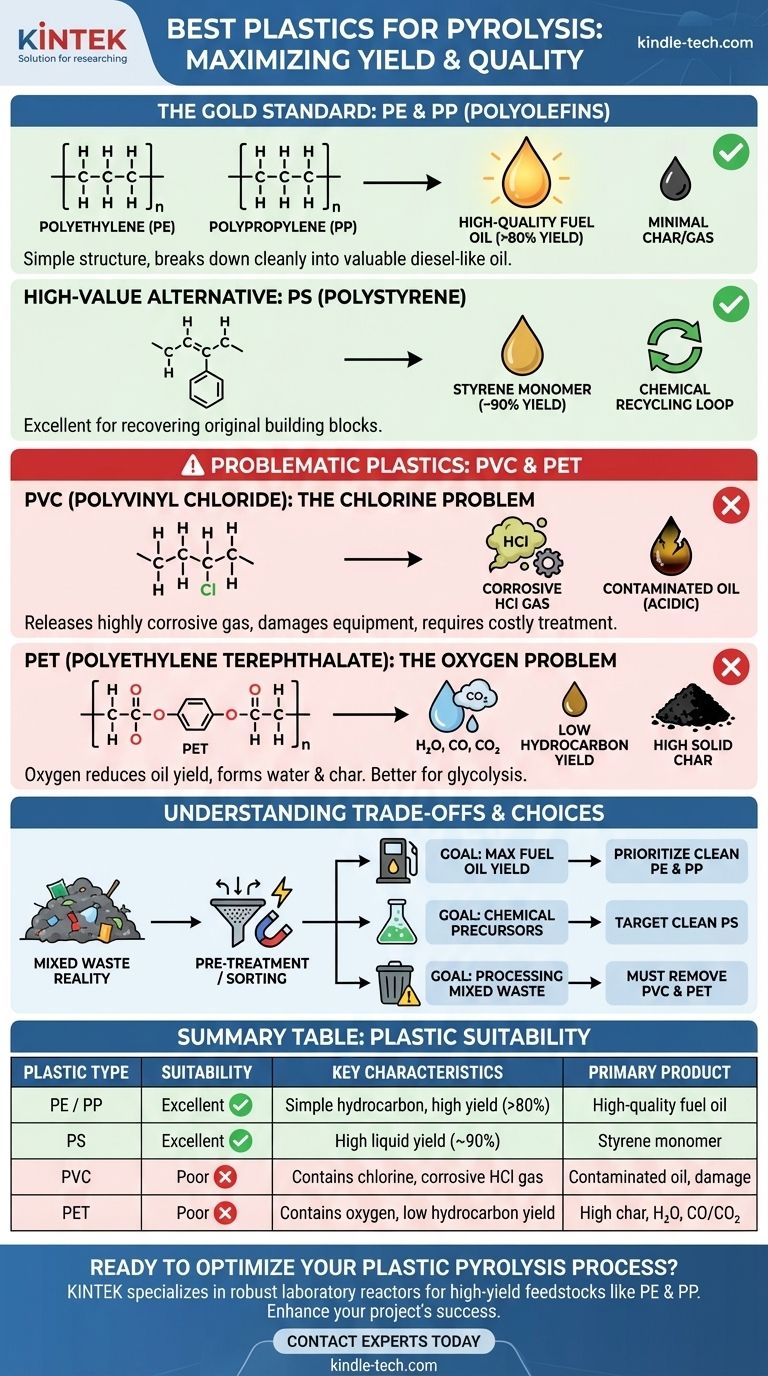
From a technical standpoint, the best plastics for pyrolysis are polyolefins, specifically Polyethylene (PE) and Polypropylene (PP), followed closely by Polystyrene (PS). These polymers are composed of simple hydrocarbon backbones, which, when heated, break down cleanly into valuable smaller hydrocarbon molecules that form a high-quality synthetic oil, similar to diesel fuel.
The ideal plastic for pyrolysis is one with a simple chemical structure, free of atoms like chlorine, oxygen, or nitrogen. These "heteroatoms" complicate the process, reduce the quality of the oil, and can create corrosive byproducts that damage equipment.

Why Polymer Structure is the Deciding Factor
The goal of pyrolysis is to thermally crack long polymer chains into smaller, more valuable liquid hydrocarbon molecules. The chemical makeup of the starting plastic directly dictates the efficiency of this process and the quality of the end products.
The Gold Standard: PE and PP
Polyethylene (HDPE, LDPE) and Polypropylene (PP) are considered the top-tier feedstocks for pyrolysis. They are simple polymers made of only carbon and hydrogen.
When heated in the absence of oxygen, their long chains break apart predictably. This process yields a very high percentage of liquid oil (often over 80% by weight) and a minimal amount of non-condensable gas and solid char. The resulting oil is rich in paraffins and olefins, making it an excellent precursor for fuels.
A High-Value Alternative: PS
Polystyrene (PS) also performs exceptionally well in pyrolysis, often producing a liquid yield of up to 90%.
However, the oil from PS is chemically different. It is rich in styrene monomer, the very chemical used to make polystyrene in the first place. This makes PS an ideal candidate for chemical recycling, where the goal is to create a circular loop by recovering the original building blocks.
Problematic Plastics and Their Challenges
While many plastics can be processed, some introduce significant technical and economic hurdles. The issues almost always stem from heteroatoms in the polymer's backbone.
The Chlorine Problem: Polyvinyl Chloride (PVC)
PVC is the most problematic plastic for pyrolysis. Its structure contains chlorine, which is released as hydrochloric acid (HCl) gas during the process.
This HCl gas is highly corrosive, causing severe damage to reactors, pipes, and condensers. It also contaminates the final oil, making it acidic and requiring costly secondary treatment steps to neutralize and remove the chlorine. Even small amounts of PVC in a mixed feedstock can render a pyrolysis operation economically unviable.
The Oxygen Problem: Polyethylene Terephthalate (PET)
PET, commonly used for beverage bottles, contains a significant amount of oxygen in its structure.
During pyrolysis, this oxygen tends to form water (H₂O), carbon monoxide (CO), and carbon dioxide (CO₂). This diverts a large portion of the plastic's mass away from a usable liquid oil, significantly lowering the hydrocarbon yield. It also produces a higher amount of solid char compared to polyolefins. For these reasons, PET is better suited for other recycling methods like glycolysis.
Understanding the Trade-offs
In a real-world scenario, you will rarely find a pure stream of a single plastic type. Feedstocks like post-consumer packaging or municipal solid waste are always mixtures.
Yield vs. Purity
The highest liquid yields come from PE, PP, and PS. However, if the feedstock is a mix, the presence of contaminants like PVC and PET will drastically lower the overall yield and degrade the final product's quality.
The Reality of Mixed Waste
For operators processing mixed plastic waste, the crucial step is pre-treatment. While the reference materials list "mixed PET/PVC contaminated plastics" as suitable, this is only true if a robust and expensive system for capturing and neutralizing acid gas is in place.
Without such a system, it is not technically or economically feasible. The most successful mixed-plastic pyrolysis operations invest heavily in sorting technologies to maximize the concentration of PE and PP while minimizing the presence of PVC.
Making the Right Choice for Your Goal
Your choice of feedstock must align directly with your operational goals and capabilities.
- If your primary focus is maximizing high-quality fuel oil yield: Prioritize clean, sorted streams of Polyethylene (PE) and Polypropylene (PP).
- If your primary focus is producing valuable chemical precursors: Target clean Polystyrene (PS) feedstock to recover styrene monomer.
- If you are processing unsorted municipal or mixed plastic waste: Your success depends on implementing a pre-sorting stage to remove as much PVC and PET as possible before it enters the reactor.
Ultimately, feedstock selection and purification are the most critical factors determining the technical success and economic viability of any plastic pyrolysis project.
Summary Table:
| Plastic Type | Pyrolysis Suitability | Key Characteristics | Primary Product |
|---|---|---|---|
| Polyethylene (PE) / Polypropylene (PP) | Excellent | Simple hydrocarbon backbone, high yield (>80%) | High-quality fuel oil |
| Polystyrene (PS) | Excellent | High liquid yield (~90%) | Styrene monomer for chemical recycling |
| Polyvinyl Chloride (PVC) | Poor | Contains chlorine, releases corrosive HCl gas | Contaminated oil, equipment damage |
| Polyethylene Terephthalate (PET) | Poor | Contains oxygen, low hydrocarbon yield | High char, water, CO/CO₂ |
Ready to optimize your plastic pyrolysis process with the right equipment? KINTEK specializes in providing robust laboratory reactors and systems designed to handle high-yield feedstocks like PE and PP efficiently. Our expertise ensures you achieve maximum oil recovery and process stability. Contact our experts today to discuss your specific needs and discover how KINTEK's solutions can enhance your pyrolysis project's success.
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Vertical Laboratory Tube Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- Why do we adopt pyrolysis process? Unlock Value from Waste with Advanced Technology
- What is the working principle of rotor furnace? Achieving Perfect Heat Treatment Uniformity
- What are the advantages of a continuous furnace? Achieve High-Volume, Consistent Thermal Processing
- What is fast and slow pyrolysis? Choosing the Right Biomass Conversion Process
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- Is pyrolysis oil hazardous? The Critical Risks of Handling This Reactive Fuel
- How does pyrolysis help the environment? Transform Waste into Renewable Energy and Carbon Sequestration
- What are the advantages of using a rotary tube furnace for MoVOx catalysts? Elevate Uniformity and Crystallinity



















