In short, the silver/silver chloride (Ag/AgCl) electrode is used as a reference electrode because it provides an exceptionally stable, predictable, and reproducible electrical potential. This stability, combined with its simple construction and relative safety compared to mercury-based alternatives, makes it the most common and reliable choice for a vast range of electrochemical measurements.
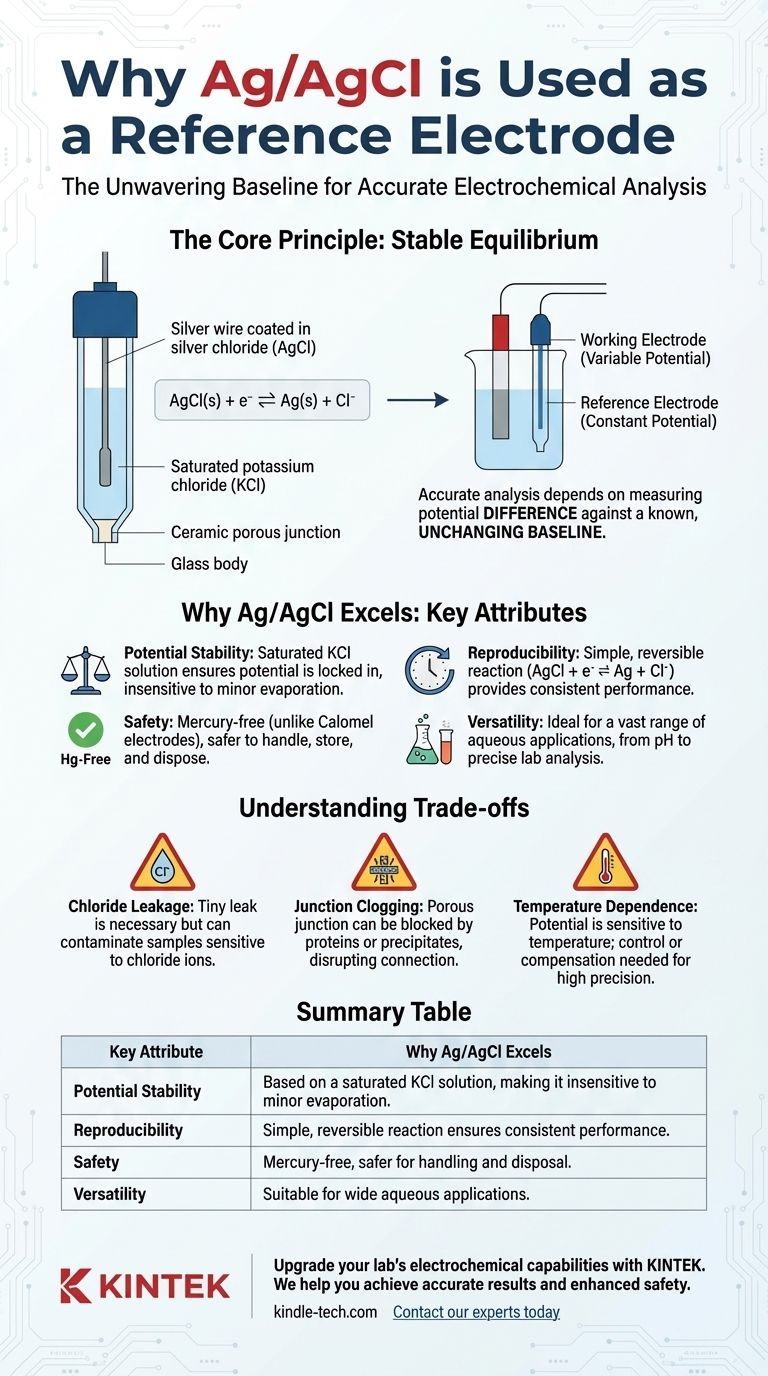
Accurate electrochemical analysis depends on measuring a variable potential against a known, unchanging baseline. The Ag/AgCl electrode provides this essential baseline by harnessing a simple chemical equilibrium that is remarkably insensitive to common environmental fluctuations.

The Foundation: What Makes a Reference Electrode Reliable?
To understand the value of the Ag/AgCl electrode, you must first understand the fundamental role of any reference electrode. It serves as the zero-point for your electrical measurement.
The Need for an Unwavering Baseline
In electrochemistry, you can only measure a potential difference between two points. One point is your sensor or working electrode, where the chemical reaction of interest occurs.
The second point must be a reference electrode. Its job is to hold a perfectly constant potential, so any change you measure in the overall system can be confidently attributed to the working electrode.
The Principle of Stable Equilibrium
A reference electrode achieves this stability by using a specific redox system (a chemical reaction involving electron transfer).
This system is designed to be "well-poised," meaning its potential is locked in. This is typically accomplished by keeping the concentrations of the chemicals involved in the reaction saturated and constant.
Because the solution concentração is saturated, small changes from evaporation or temperature shifts have a negligible effect on the electrode's potential, ensuring it remains a stable reference point.
Why Ag/AgCl Excels in This Role
The Ag/AgCl electrode is not just one option among many; it is the industry standard for most applications because it masterfully embodies these core principles.
Predictable and Stable Potential
The Ag/AgCl electrode is built around a simple, highly reversible reaction: AgCl(s) + e⁻ ⇌ Ag(s) + Cl⁻
The potential of the electrode is determined by the activity (effectively, the concentration) of the chloride ions (Cl⁻) in the internal filling solution.
By using a solution with a fixed, known concentration of potassium chloride (KCl)—often a saturated solution—the potential is locked in and becomes a reliable constant.
Robustness Against Environmental Changes
The use of a saturated KCl fill solution is a key design feature. If any water evaporates from the solution, the KCl concentration does not change because the solution was already saturated; some solid KCl will simply precipitate out.
This makes the electrode's potential remarkably stable over time and across a wider temperature range than alternatives like the Saturated Calomel Electrode (SCE).
Simple, Safe, and Versatile Construction
An Ag/AgCl electrode consists of a silver wire coated with a layer of silver chloride, immersed in the KCl fill solution. This assembly is contained within a glass or epoxy body.
A porous junction, often made of ceramic, allows a tiny, controlled amount of the fill solution to leak into the sample. This leakage is essential to complete the electrical circuit.
Critically, this construction avoids the use of toxic mercury, which is found in calomel electrodes, making Ag/AgCl safer to handle, store, and dispose of.
Understanding the Trade-offs and Pitfalls
While Ag/AgCl is a superior general-purpose electrode, it is not without limitations. Understanding these is crucial for accurate measurements.
The Problem of Chloride Leakage
The tiny leak from the junction is necessary, but it also means that chloride ions from the fill solution will enter your sample.
If your sample contains species that react with or are sensitive to chloride (such as silver ions), this contamination can cause significant measurement errors or precipitate solids that clog the junction.
Junction Clogging
The porous junction is the most common point of failure. It can become clogged by proteins, colloids, or precipitates from the sample, disrupting the electrical connection.
A clogged junction leads to slow, noisy, and drifting readings, rendering measurements unreliable. Regular cleaning and proper storage are essential to prevent this.
Temperature Dependence
While stable, the Ag/AgCl potential is not perfectly immune to temperature. Both the electrode reaction and the solubility of KCl are temperature-dependent.
For high-precision work, measurements must be performed at a constant temperature, or temperature compensation must be applied.
Making the Right Choice for Your Application
Your choice of reference electrode configuration should be guided by your specific analytical goal.
- If your primary focus is general lab work (e.g., pH): A standard, single-junction Ag/AgCl electrode with saturated KCl is the default choice for its stability, reliability, and cost-effectiveness.
- If your sample is sensitive to chloride or potassium ions: You must use a double-junction electrode, which has a second outer chamber with a more compatible electrolyte to prevent contamination.
- If you are working in non-aqueous solvents: A standard Ag/AgCl electrode is unsuitable, and you will need a specialized reference electrode designed for your specific solvent system.
- If you require the highest possible accuracy: Always calibrate your system frequently and control the temperature of your measurement to minimize drift.
By understanding these principles, the Ag/AgCl reference electrode becomes a predictable and powerful component of your analytical system.
Summary Table:
| Key Attribute | Why Ag/AgCl Excels |
|---|---|
| Potential Stability | Based on a saturated KCl solution, making it insensitive to minor evaporation or temperature changes. |
| Reproducibility | Simple, reversible reaction (AgCl + e⁻ ⇌ Ag + Cl⁻) ensures consistent performance across electrodes. |
| Safety | Mercury-free, unlike calomel electrodes, making it safer for handling and disposal. |
| Versatility | Suitable for a wide range of aqueous applications, from pH measurement to precise lab analysis. |
Upgrade your lab's electrochemical capabilities with KINTEK.
Whether you're conducting precise pH measurements, electrochemical analysis, or routine lab work, the right reference electrode is critical for accuracy. KINTEK specializes in high-quality lab equipment and consumables, including reliable Ag/AgCl reference electrodes designed for stability and longevity.
We help you achieve:
- Accurate and reproducible results with electrodes built for consistent performance.
- Enhanced lab safety with mercury-free alternatives.
- Tailored solutions for specific applications, including double-junction designs for chloride-sensitive samples.
Don't let electrode instability compromise your data. Contact our experts today to find the perfect reference electrode for your laboratory needs and ensure your measurements are always on point.
Visual Guide
Related Products
- Reference Electrode Calomel Silver Chloride Mercury Sulfate for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Copper Sulfate Reference Electrode for Laboratory Use
- RRDE rotating disk (ring disk) electrode / compatible with PINE, Japanese ALS, Swiss Metrohm glassy carbon platinum
- Platinum Sheet Electrode for Laboratory and Industrial Applications
People Also Ask
- How should a pre-treated carbon fiber brush be installed? Ensure Reliable Electrochemical Performance
- What are the post-treatment procedures after using a copper sulfate reference electrode? Essential Steps for Accuracy & Longevity
- How do three-dimensional metal foam cathodes improve metal electrowinning efficiency? Triple Your Deposition Rates
- How can mechanical damage to a platinum wire electrode be prevented? Essential Tips for Accurate Electrochemistry
- What are the key precautions to take when using titanium electrodes? Avoid Costly Damage and Maximize Performance
- What are the reasons for selecting a platinum electrode as the counter electrode? Ensure Pure Data in Duplex Steel Tests
- Why can graphite withstand heat? Unlocking Its Extreme Thermal Stability for Your Lab
- How does a saturated Ag/AgCl reference electrode ensure accuracy in Ni-Cr alloy tests? Master High-Temp Precision



















