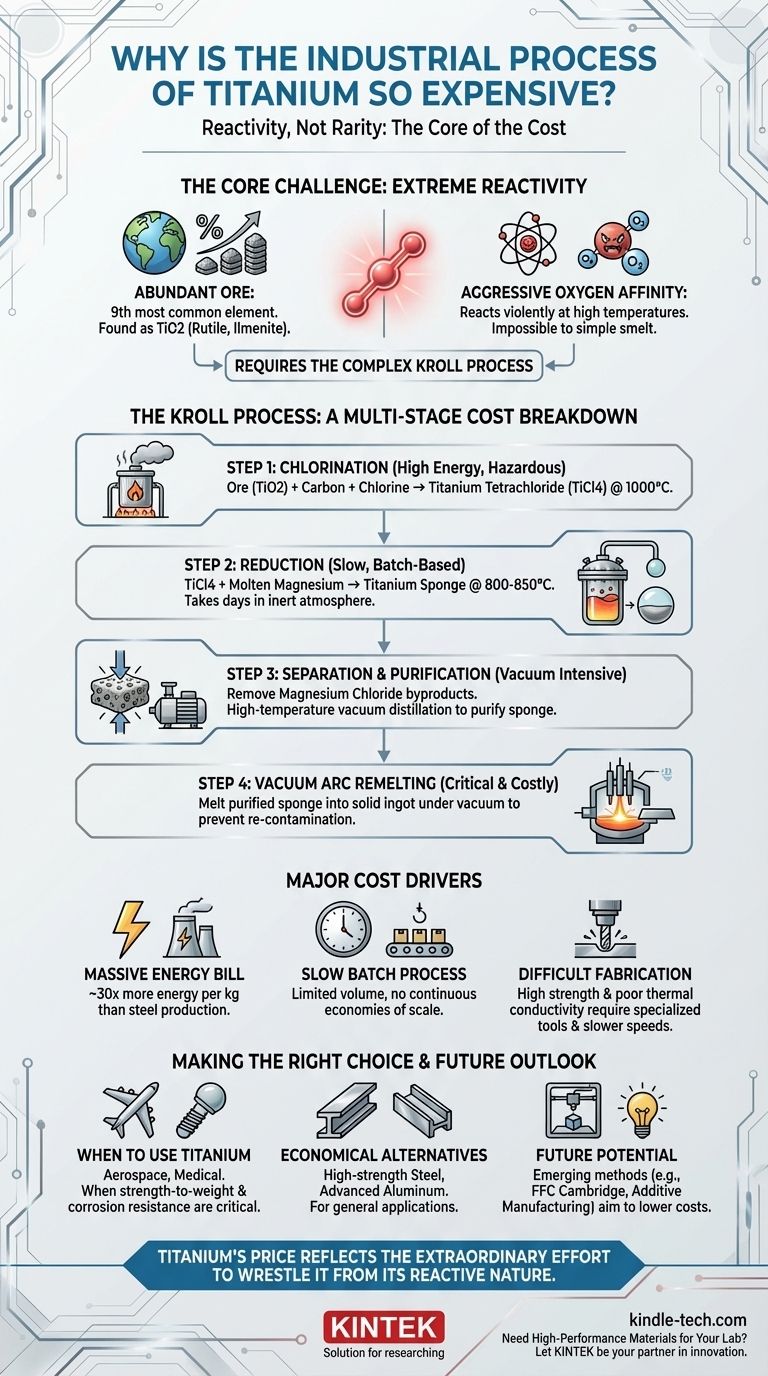
At its core, the immense expense of producing titanium is not due to the rarity of its ore but because of its extreme chemical reactivity at high temperatures. This reactivity makes traditional, cost-effective smelting impossible and forces the industry to use a complex, multi-stage, and highly energy-intensive method known as the Kroll process.
The fundamental reason titanium is so expensive is that it cannot be produced using simple, continuous smelting like steel. Its aggressive affinity for oxygen requires a slow, batch-based process involving hazardous chemicals, inert atmospheres, and extreme energy consumption to protect the metal from contamination.

The Challenge: Titanium's Aggressive Chemistry
To understand the cost, you must first understand the unique chemical problem titanium presents. It is fundamentally different from iron or aluminum.
Abundant but Tightly Bound
Titanium is the ninth most abundant element in the Earth's crust, far more common than copper, lead, or tin. It is typically found as titanium dioxide (TiO2) in minerals like rutile and ilmenite.
The problem isn't finding it; it's breaking the powerful chemical bond between titanium and oxygen in its natural ore.
An Unquenchable Thirst for Oxygen
At the high temperatures needed for metal extraction, titanium reacts aggressively with oxygen, nitrogen, and hydrogen in the air.
Attempting to smelt titanium ore in a traditional blast furnace, as is done with iron, would be a failure. Instead of producing pure metal, you would create brittle, useless titanium oxides and nitrides.
Why Steel Smelting is So Much Cheaper
Iron production is a model of efficiency. Iron ore is mixed with carbon (coke) and limestone in a blast furnace, and hot air is blown through it. The carbon strips the oxygen from the iron in a continuous, large-scale, and relatively simple process.
Titanium’s reactivity completely prevents this direct and economical approach. It must be shielded from oxygen at every high-temperature stage of production.
The Kroll Process: A Step-by-Step Cost Breakdown
The Kroll process, developed in the 1940s, is the primary industrial method for producing titanium. It is a slow, multi-step batch process that directly contributes to the metal's high cost.
Step 1: Creating Titanium Tetrachloride (TiCl4)
The process begins by converting solid titanium dioxide (TiO2) into a liquid. The ore is heated to around 1,000°C in a reactor with carbon and chlorine gas.
This reaction produces titanium tetrachloride (TiCl4), a volatile and highly corrosive liquid. This step alone is energy-intensive and involves handling hazardous materials.
Step 2: The Reduction Phase
The purified TiCl4 is moved to a sealed stainless steel reactor. All the air is pumped out and replaced with an inert gas, typically argon.
Molten magnesium is then added to the reactor as a reducing agent. At high temperatures (800-850°C), the magnesium strips the chlorine atoms from the TiCl4, leaving behind pure titanium metal. This reaction is extremely slow, often taking several days to complete.
Step 3: Separating the "Titanium Sponge"
The result is not a liquid metal but a porous, solid mass called a "titanium sponge," which is intermixed with the byproduct, magnesium chloride (MgCl2), and unreacted magnesium.
Step 4: Purification and Melting
This mixture is crushed, and the magnesium chloride is separated out. The remaining titanium sponge is then purified through a high-temperature vacuum distillation process to remove any remaining magnesium.
Finally, the purified sponge is crushed, blended, and melted into a solid ingot. This melting must be done in a Vacuum Arc Remelting (VAR) furnace to, once again, prevent any contamination from atmospheric oxygen.
Understanding the Trade-offs and Additional Costs
The complexity of the Kroll process creates cascading costs that extend beyond the primary production steps.
The Massive Energy Bill
Each stage—chlorination, reduction, vacuum distillation, and vacuum arc melting—consumes vast amounts of energy. Producing one kilogram of titanium requires roughly 30 times more energy than producing one kilogram of common steel.
The Batch Process Limitation
Unlike the continuous flow of a steel mill, the Kroll process is a series of discrete, slow batches. This inherently limits production volume, increases labor costs per unit, and prevents the economies of scale seen in steel and aluminum production.
Difficult Downstream Fabrication
The high cost doesn't end when the ingot is formed. Titanium's poor thermal conductivity and high strength make it notoriously difficult to machine. It requires specialized cutting tools, slower processing speeds, and extensive coolants, adding significant cost to the final fabricated part.
The Quest for a Cheaper Method
For decades, researchers have sought a more direct and cost-effective alternative to the Kroll process.
Promising Electrochemical Alternatives
Processes like the FFC Cambridge process aim to directly reduce solid titanium dioxide into titanium metal using electrolysis in a molten salt bath. In theory, this could be a simpler, continuous, and less energy-intensive route.
Why the Kroll Process Endures
Despite these promising alternatives, the Kroll process has been refined for over 70 years and is proven to produce the extremely high-purity titanium required for critical aerospace and medical applications. The industrial and financial challenge of scaling a new technology to meet this trusted standard has so far prevented a widespread replacement.
Making the Right Material Choice
Understanding these production hurdles is key to deciding when and how to use titanium.
- If your primary focus is ultimate strength-to-weight and corrosion resistance: The high cost of Kroll-processed titanium is justified for demanding applications where performance is non-negotiable, such as in aerospace or medical implants.
- If your primary focus is cost-effectiveness for general applications: High-strength steel or advanced aluminum alloys will almost always be the more economical and practical choice.
- If you are exploring next-generation manufacturing: Keep a close watch on emerging production methods, as they hold the potential to disrupt the cost structure, particularly for applications like additive manufacturing (3D printing).
Ultimately, titanium's price is a direct reflection of the extraordinary chemical and engineering efforts required to wrestle it from its natural, oxidized state into a pure, usable metal.
Summary Table:
| Cost Factor | Reason for High Cost |
|---|---|
| Ore Processing | Cannot be smelted like iron; requires conversion to volatile TiCl4. |
| Reduction Process | Slow, batch-based Kroll process using molten magnesium in inert atmosphere. |
| Energy Consumption | Requires ~30x more energy per kg than steel production. |
| Purification & Melting | Needs vacuum distillation and Vacuum Arc Remelting (VAR) to prevent contamination. |
| Fabrication | Difficult to machine due to high strength and poor thermal conductivity. |
Need High-Performance Materials for Your Lab?
The challenges of working with reactive metals like titanium demand reliable, high-quality equipment. KINTEK specializes in supplying the robust lab equipment and consumables needed for advanced material processing and research. Whether you're developing new alloys or analyzing material properties, our solutions support precision and safety in demanding environments.
Let KINTEK be your partner in innovation. Contact our experts today to discuss how our products can meet your specific laboratory needs.
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vacuum Dental Porcelain Sintering Furnace
People Also Ask
- Why would you braze instead of weld? Preserve Material Integrity and Join Dissimilar Metals
- What is the cost of a vacuum brazing furnace? A guide to key factors and investment strategy
- Can dissimilar metals be brazed or braze welded? A Guide to Strong, Reliable Joints
- Does brazing require heat? Yes, it's the catalyst for creating strong, permanent bonds.
- What is the major advantage that brazing has over welding? Joining Dissimilar Metals with Ease



















