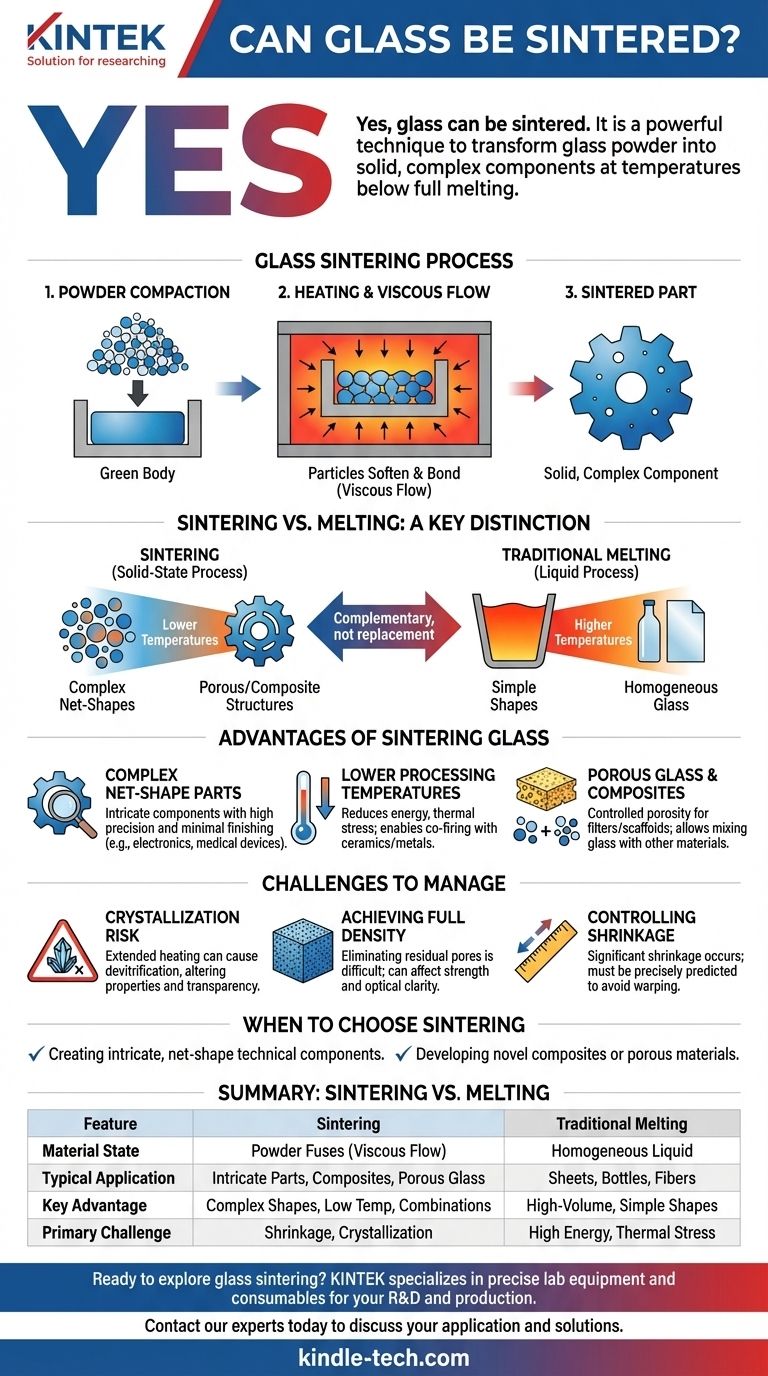
Yes, glass can be sintered. It is a well-established and powerful manufacturing technique used to transform glass powder into solid, complex components. The process involves heating compacted glass particles to a temperature high enough to cause them to fuse together, but below the point where the glass fully melts into a liquid.
While traditional glassmaking relies on complete melting, sintering provides a crucial alternative. It enables the fabrication of intricate glass shapes, composites, and porous structures at lower temperatures by bonding powdered particles, fundamentally expanding the material's applications.

What Sintering Means for Glass
Sintering is not a replacement for traditional glass melting but a complementary process for specialized applications. Understanding its mechanism is key to appreciating its value.
The Fundamental Process
In sintering, fine glass powder, often called "frit," is first compacted into a desired shape. This "green body" is then heated in a furnace. As the temperature rises, the viscosity of the glass decreases, and surface tension pulls the particles together, causing them to fuse and densify the structure.
Sintering vs. Melting: A Key Distinction
The defining difference is the state of the material. Melting involves heating glass until it becomes a homogeneous liquid, which is then cast, blown, or drawn into a shape. Sintering is a solid-state (or more accurately, viscous-flow) process that bonds particles together without ever reaching a fully liquid phase.
The Driving Force: Viscous Flow
For glass, the primary mechanism of sintering is viscous flow. Unlike the atomic diffusion that drives sintering in metals and crystalline ceramics, glass particles soften and flow under heat, allowing them to merge and eliminate the pores between them.
The Advantages of Sintering Glass
Engineers and material scientists turn to sintering when conventional methods fall short. The process unlocks several unique capabilities.
Fabricating Complex, Net-Shape Parts
Sintering excels at producing small, intricate components with high precision. Because the material starts as a powder, it can be molded into a "net shape" that requires little to no finishing, a task that is extremely difficult or impossible with molten glass. This is critical for electronics, optics, and medical device components.
Lower Processing Temperatures
Sintering occurs at temperatures significantly lower than those needed to melt glass. This reduces energy consumption and thermal stress on equipment. More importantly, it allows glass to be co-fired with other materials, like ceramics or metals, that could not withstand the high temperatures of glass melting.
Creating Porous Glass and Composites
By controlling the sintering time and temperature, you can stop the process before full densification occurs. This is used to intentionally create porous glass, which serves as filters, vents, or biomedical scaffolds. It is also the only practical way to create glass-matrix composites by mixing glass powder with other powdered materials.
Understanding the Trade-offs and Challenges
While powerful, glass sintering is a technical process with specific challenges that must be managed to achieve desired outcomes.
The Risk of Unwanted Crystallization
Glass is an amorphous, non-crystalline solid. However, holding it at an elevated temperature for an extended period—as is done during sintering—can cause it to devitrify, or form crystalline regions. This can alter its mechanical properties, chemical resistance, and, most notably, its transparency.
Achieving Full Density
Eliminating the last few percent of porosity can be very difficult. Residual pores can act as stress concentrators, reducing the mechanical strength of the final part. For optical applications, these pores can scatter light, making them highly undesirable.
Controlling Shrinkage
As the powder compact densifies, it shrinks. This shrinkage can be substantial (15-20% or more) and must be precisely predicted and controlled to achieve the final desired dimensions and tolerances. Non-uniform shrinkage can lead to warping or cracking.
Applying Glass Sintering to Your Goal
Choosing between sintering and traditional melting depends entirely on your final objective and the complexity of the component you need.
- If your primary focus is creating intricate, net-shape components for technical applications: Sintering is the superior method as it bypasses the forming limitations of molten glass.
- If your primary focus is developing novel composites or porous materials: Sintering is often the only viable path, allowing you to combine glass with other materials or engineer controlled porosity.
- If your primary focus is producing simple, high-volume shapes like sheets, bottles, or fibers: Traditional melting and forming remains the most established and cost-effective process.
By understanding sintering as a specialized tool in materials processing, you can unlock a new range of possibilities for advanced glass fabrication.
Summary Table:
| Feature | Sintering | Traditional Melting |
|---|---|---|
| Material State | Powdered particles fuse (viscous flow) | Homogeneous liquid |
| Typical Application | Intricate components, composites, porous glass | Sheets, bottles, fibers |
| Key Advantage | Complex net-shapes, lower temperatures, material combinations | High-volume, simple shapes |
| Primary Challenge | Controlling shrinkage, avoiding crystallization | High energy consumption, thermal stress |
Ready to explore how sintering can advance your glass fabrication projects?
KINTEK specializes in providing the precise lab equipment and consumables needed for successful glass sintering R&D and production. Our expertise helps you overcome challenges like shrinkage control and crystallization to achieve your material goals—from intricate medical device components to novel glass-matrix composites.
Contact our experts today to discuss your specific application and discover the right solutions for your laboratory.
Visual Guide
Related Products
- Vacuum Dental Porcelain Sintering Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- What is the difference between VAR and VIM? Legacy Vimscript Variables vs. Modern Neovim API
- What temperature is porcelain fired at? A Guide to Precise Dental Firing Cycles
- What are five applications of soldering? From Electronics to Art, Master Material Joining
- Why are porcelain fired under vacuum? To Eliminate Porosity for Superior Strength & Translucency
- Can high fusing porcelain be repaired without distortion? Yes, with the right low-fusing materials and techniques.



















