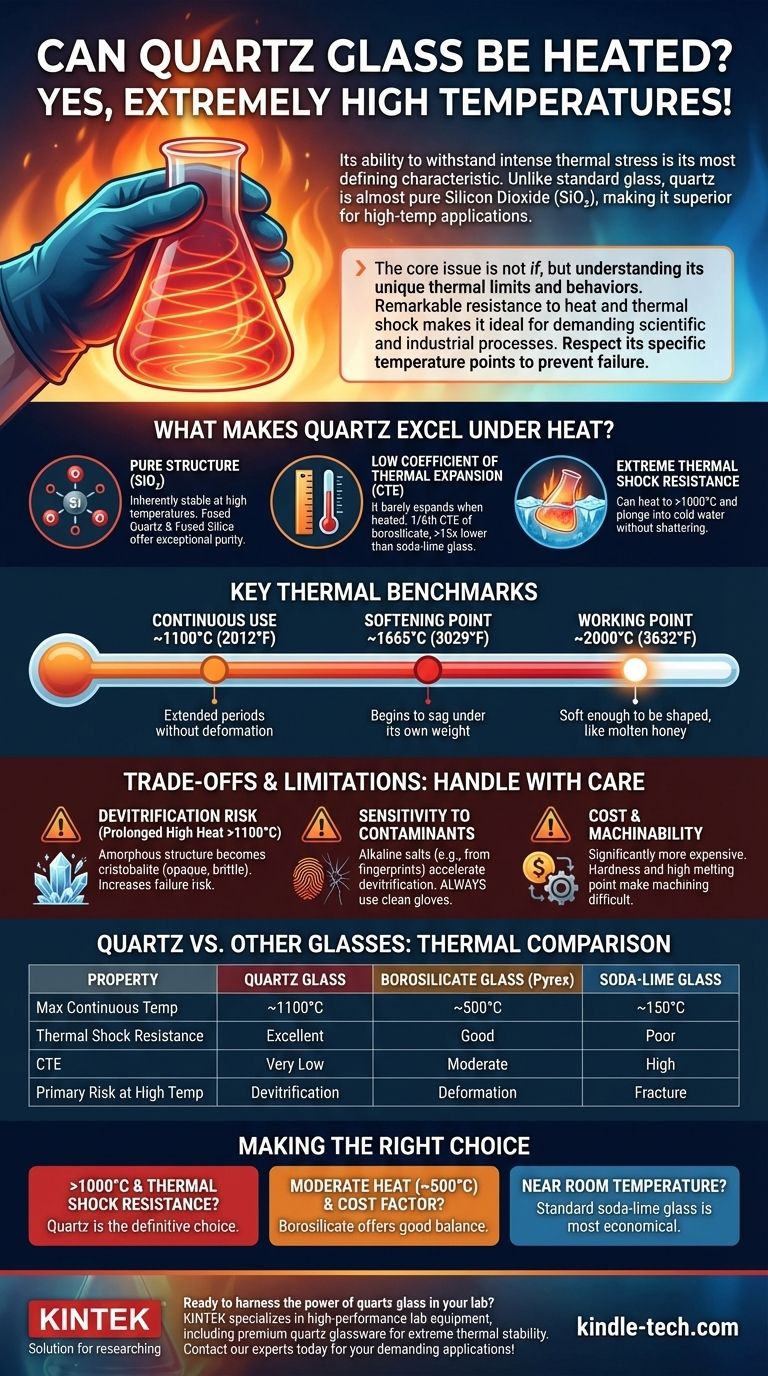
Yes, quartz glass can be heated to extremely high temperatures. In fact, its ability to withstand intense thermal stress is its most defining characteristic. Unlike standard glass, quartz is composed of almost pure silicon dioxide (SiO₂), giving it an exceptionally low coefficient ofthermal expansion and a very high melting point, making it a superior material for high-temperature applications.
The core issue is not if quartz can be heated, but understanding its unique thermal limits and behaviors. Its remarkable resistance to heat and thermal shock makes it ideal for demanding scientific and industrial processes, but you must respect its specific temperature points to prevent material failure through devitrification.

What Makes Quartz Excel Under Heat?
The thermal superiority of quartz glass is not magic; it stems directly from its fundamental chemical structure and purity. Understanding these principles is key to using it effectively.
The Role of Purity
Quartz glass is essentially pure amorphous silicon dioxide. This simple and strong molecular structure is inherently stable at high temperatures.
Materials sold as "quartz glass" fall into two main categories: fused quartz, made by melting natural quartz crystals, and fused silica, made from high-purity synthetic silicon compounds. Fused silica offers higher purity and even better thermal performance.
Exceptionally Low Coefficient of Thermal Expansion (CTE)
The single most important property is its incredibly low Coefficient of Thermal Expansion (CTE). This measures how much a material expands or contracts when its temperature changes.
Quartz has a CTE that is roughly 1/6th that of borosilicate glass (like Pyrex) and over 15 times lower than standard soda-lime glass.
This means when quartz is heated, it barely expands. This stability is why you can heat it to over 1000°C and plunge it into cold water without it cracking—an action that would instantly shatter almost any other type of glass.
Key Thermal Benchmarks
To use quartz safely, you must know its operational temperature limits. These aren't single numbers but rather ranges where the material's behavior changes.
- Continuous Use Temperature: Up to ~1100°C (2012°F). Quartz can be held at this temperature for extended periods without significant deformation.
- Softening Point: Approximately 1665°C (3029°F). At this temperature, the glass begins to sag under its own weight.
- Working Point: Around 2000°C (3632°F). The glass becomes soft enough to be shaped and worked, similar to molten honey.
Understanding the Trade-offs and Limitations
While powerful, quartz is not indestructible. Its primary failure mode at high temperature is not melting but a structural change that compromises its integrity.
The Risk of Devitrification
If held at high temperatures (especially above 1100°C) for prolonged periods, quartz can begin to devitrify. This is a process where the amorphous (glassy) structure crystallizes into a form called cristobalite.
This crystallized form is opaque, brittle, and has a much higher CTE. Devitrification makes the material weak and prone to failure from thermal stress.
Sensitivity to Surface Contaminants
Devitrification is significantly accelerated by surface contaminants, especially alkaline salts. Even the oils and salts from a fingerprint can act as a catalyst for crystallization at high temperatures.
For this reason, any quartz component intended for high-heat use must be handled with clean gloves and be thoroughly cleaned before being put into service.
Cost and Machinability
The exceptional properties of quartz come at a price. It is significantly more expensive than borosilicate or soda-lime glass. Furthermore, its hardness and high melting point make it more difficult and costly to machine and form into complex shapes.
Quartz vs. Other Glasses: A Thermal Comparison
Context is crucial. Seeing how quartz stacks up against other common lab and industrial glasses highlights its unique value proposition.
vs. Borosilicate Glass (e.g., Pyrex)
Borosilicate glass is known for its decent thermal shock resistance, making it common for labware and bakeware. However, its maximum short-term use temperature is only around 500°C (932°F). It is a good mid-range option but cannot compete with quartz in high-temperature environments.
vs. Soda-Lime Glass (Standard Glass)
This is the glass used for windows and bottles. It has a very high CTE and almost no resistance to thermal shock. Its maximum operating temperature is only around 150°C (302°F) before it becomes highly susceptible to fracture from thermal stress.
Making the Right Choice for Your Application
Selecting the correct glass is a matter of matching the material's properties to the thermal demands of your task.
- If your primary focus is extreme temperature stability (>1000°C) and thermal shock resistance: Quartz is the definitive choice, unmatched by other common glasses.
- If your application involves moderate heat (up to 500°C) and cost is a major factor: Borosilicate glass offers a good balance of thermal performance and affordability.
- If your application operates near room temperature with no thermal demands: Standard soda-lime glass is the most economical option.
By understanding these material properties, you are empowered to select the precise tool for your thermal challenge.
Summary Table:
| Property | Quartz Glass | Borosilicate Glass | Soda-Lime Glass |
|---|---|---|---|
| Max Continuous Use Temp | ~1100°C (2012°F) | ~500°C (932°F) | ~150°C (302°F) |
| Thermal Shock Resistance | Excellent | Good | Poor |
| Coefficient of Thermal Expansion | Very Low | Moderate | High |
| Primary Risk at High Temp | Devitrification | Deformation | Fracture |
Ready to harness the power of quartz glass in your lab? At KINTEK, we specialize in providing high-performance lab equipment and consumables, including premium quartz glassware designed for extreme thermal stability. Whether you're working in materials science, semiconductor manufacturing, or high-temperature research, our quartz products ensure reliability and precision. Don't compromise on quality—contact our experts today to find the perfect quartz solution for your demanding applications!
Visual Guide
Related Products
- Optical Window Glass Substrate Wafer Single Double Sided Coated K9 Quartz Sheet
- Optical Ultra-Clear Glass Sheet for Laboratory K9 B270 BK7
- 400-700nm Wavelength Anti Reflective AR Coating Glass
- Zinc Selenide ZnSe Optical Window Glass Substrate Wafer and Lens
- Automatic Laboratory Heat Press Machine
People Also Ask
- What is a polishing pad made of and how should it be used and maintained? Master the Art of a Perfect Finish
- What is the purpose of a water-cooled condenser in tubular furnace reduction? Protect Your Lab and Equipment.
- What is the function of a magnetic stirrer in SiO2FexOy synthesis? Achieve Molecular Homogeneity in Sol-Gel Processes
- Why are high-temperature resistant springs used for Tantalum wire in HFCVD? Achieve Precise Diamond Film Uniformity
- What are the advantages of using zirconia milling jars for sulfide electrolytes? Enhance Purity and Conductivity
- How do high-precision PID controllers ensure the accuracy of process optimization data? Master Dynamic Temperature Ramps
- Why are zirconia grinding balls preferred for the grinding process of Ni-SmDC catalyst powders? Ensuring Peak Purity
- How does graphite paper function as a consumable in hot pressing? Essential Tooling Protection for EHEA Composites



















