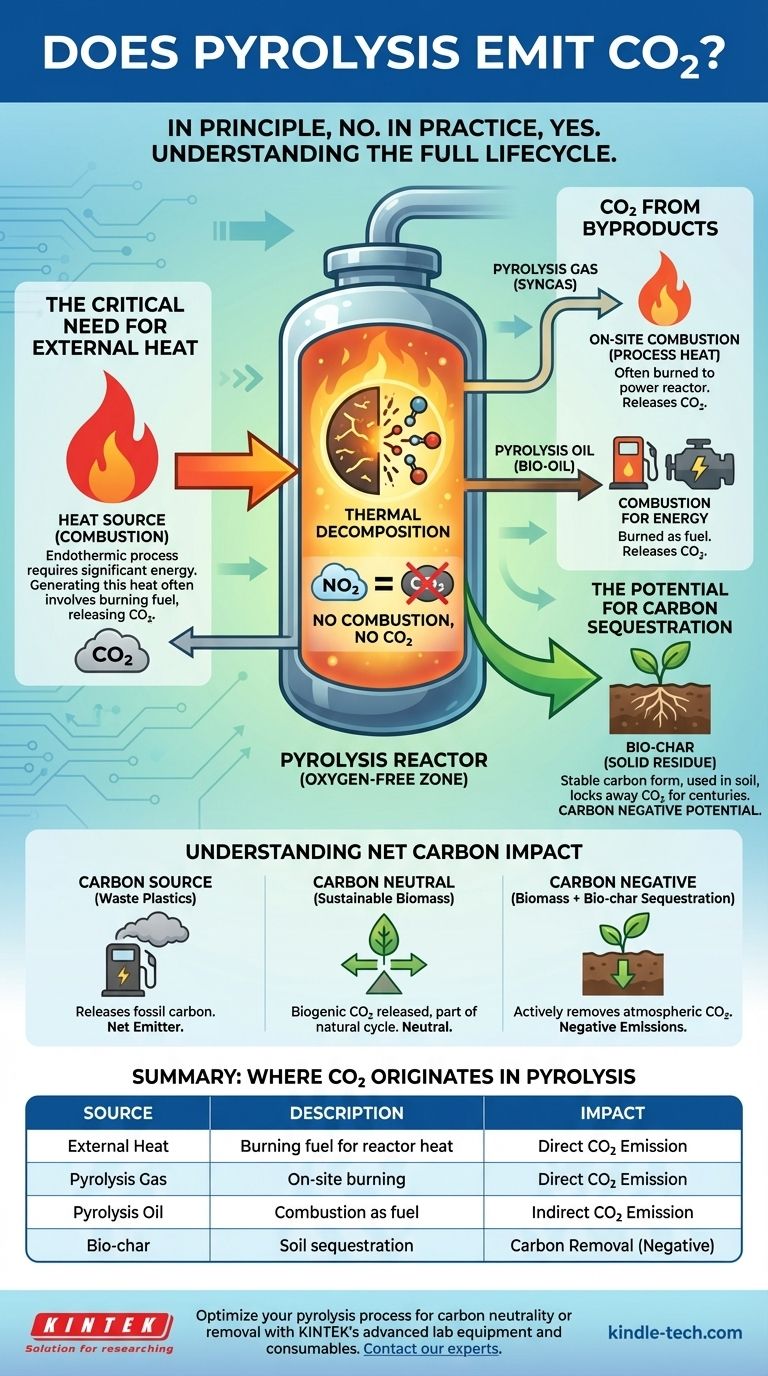
In principle, no, but in practice, yes. The core chemical reaction of pyrolysis—the thermal decomposition of material in an oxygen-free environment—does not produce carbon dioxide (CO2) through combustion. However, the overall pyrolysis process is an energy-intensive system that almost always results in CO2 emissions, primarily from generating the required heat and from the subsequent use of its carbon-based products.
While the core pyrolysis reaction itself is anaerobic and avoids direct combustion, a complete pyrolysis facility is not CO2-free. Emissions are an inherent part of the larger system, generated by the energy required to heat the reactor and by the eventual combustion of the gas and oil products.

Where CO2 Originates in a Pyrolysis System
To understand the carbon footprint of pyrolysis, you must look beyond the central reaction chamber and analyze the entire operational lifecycle.
The Core Reaction: An Oxygen-Free Zone
Pyrolysis is fundamentally different from incineration (burning). It heats feedstock, such as biomass or plastic, to high temperatures in the absence of oxygen.
Without oxygen, the material cannot combust. Instead, it breaks down chemically into smaller, different molecules. This is why the core reaction itself does not release the feedstock's carbon as CO2.
The Critical Need for External Heat
Pyrolysis is an endothermic process, meaning it requires a constant and significant input of energy to maintain the high temperatures needed for decomposition.
This heat must be generated somehow. In most industrial plants, this is achieved by burning a fuel source, which is a process of combustion that releases CO2.
The Carbon in the Byproducts
The pyrolysis reaction transforms the initial feedstock into three main products, all of which contain carbon. The fate of these products determines the final CO2 impact.
Pyrolysis Gas (Syngas)
This non-condensable gas mixture often contains carbon monoxide (CO), hydrogen (H2), methane (CH4), and some CO2.
Most modern pyrolysis plants are designed to be self-sustaining. They burn this pyrolysis gas on-site to generate the heat needed to run the reactor. This combustion converts the CO and CH4 into CO2.
Pyrolysis Oil (Bio-oil)
This liquid product is a dense, carbon-rich fuel. It can be stored, transported, and used as an alternative to conventional fuel oil or refined further.
When this oil is eventually burned for energy, the carbon it contains is released as CO2, similar to any other hydrocarbon fuel.
Bio-char (Solid Residue)
Bio-char is a stable, solid material that is rich in carbon. This is the product that gives pyrolysis its unique environmental potential.
Unlike the gas and oil, which are typically burned, bio-char can be used as a soil amendment in agriculture. When added to soil, its carbon is sequestered, meaning it is locked away from the atmosphere for hundreds or even thousands of years.
Understanding the Net Carbon Impact
The question of whether pyrolysis is "good" or "bad" for the climate depends entirely on the feedstock you start with and the way you use the products.
When Pyrolysis is a Carbon Source
If you use fossil-fuel-based feedstock like waste plastics and burn all the resulting oil and gas for energy, the process is a net emitter of CO2. You are simply taking fossil carbon and releasing it into the atmosphere through a different pathway.
The Path to Carbon Neutrality
If the feedstock is sustainable biomass (like agricultural waste or forestry residues), the process can be considered carbon neutral.
The CO2 released from heating the reactor or burning the bio-oil is biogenic—it's part of the short-term carbon cycle. This is carbon that the plant absorbed from the atmosphere as it grew, and it would have been released anyway when the plant decomposed naturally.
The Potential for Carbon Sequestration
The most powerful application of pyrolysis is for carbon removal. When biomass is used as a feedstock and the resulting bio-char is permanently sequestered in the soil, the process becomes carbon negative.
This technology actively takes CO2 that was recently in the atmosphere (captured by the plant) and locks it into a stable, solid form, effectively removing it from the carbon cycle.
Making the Right Choice for Your Goal
The carbon impact of pyrolysis is not a fixed value; it is a direct consequence of your specific goals and operational choices.
- If your primary focus is waste-to-energy conversion: Pyrolysis is an effective method, but you must account for the CO2 that will be emitted when the resulting fuels are inevitably burned.
- If your primary focus is producing sustainable fuels: Using biomass as a feedstock allows you to create carbon-neutral fuels, as the released CO2 is part of the existing biogenic carbon cycle.
- If your primary focus is active carbon removal: Pyrolysis of biomass specifically to create and sequester bio-char is one of the most promising and scalable technologies for drawing down atmospheric CO2.
Ultimately, the carbon footprint of a pyrolysis system is determined entirely by the feedstock used and how its valuable products are managed.
Summary Table:
| Source of CO2 | Description | Impact |
|---|---|---|
| External Heat Generation | Burning fuel to power the reactor's high temperatures. | Direct CO2 emission. |
| Pyrolysis Gas (Syngas) Combustion | On-site burning of gas for process heat. | Direct CO2 emission. |
| Pyrolysis Oil (Bio-oil) Use | Combustion of oil as fuel elsewhere. | Indirect CO2 emission. |
| Bio-char Sequestration | Using bio-char as a soil amendment. | Carbon removal (negative emissions). |
Ready to optimize your pyrolysis process for carbon neutrality or carbon removal? KINTEK specializes in advanced lab equipment and consumables for pyrolysis research and development. Whether you're testing feedstocks, analyzing bio-char, or scaling up sustainable fuel production, our precise tools help you achieve accurate, reliable results. Contact our experts today to discuss how we can support your laboratory's specific needs in waste-to-energy and carbon sequestration technologies.
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- How does a quartz tube vacuum furnace contribute to the crystallization process of Ag-doped Li-argyrodite electrolytes?
- How do a quartz tube reactor and atmosphere furnace collaborate in Co@NC pyrolysis? Master Precision Synthesis
- How is the temperature in a tube furnace measured and controlled? Master Precise Thermal Processing
- How to clean a tube furnace? A Step-by-Step Guide for Safe and Effective Maintenance
- What precautions should be taken when using a tube furnace? Ensure Safe, Effective High-Temperature Processing



















