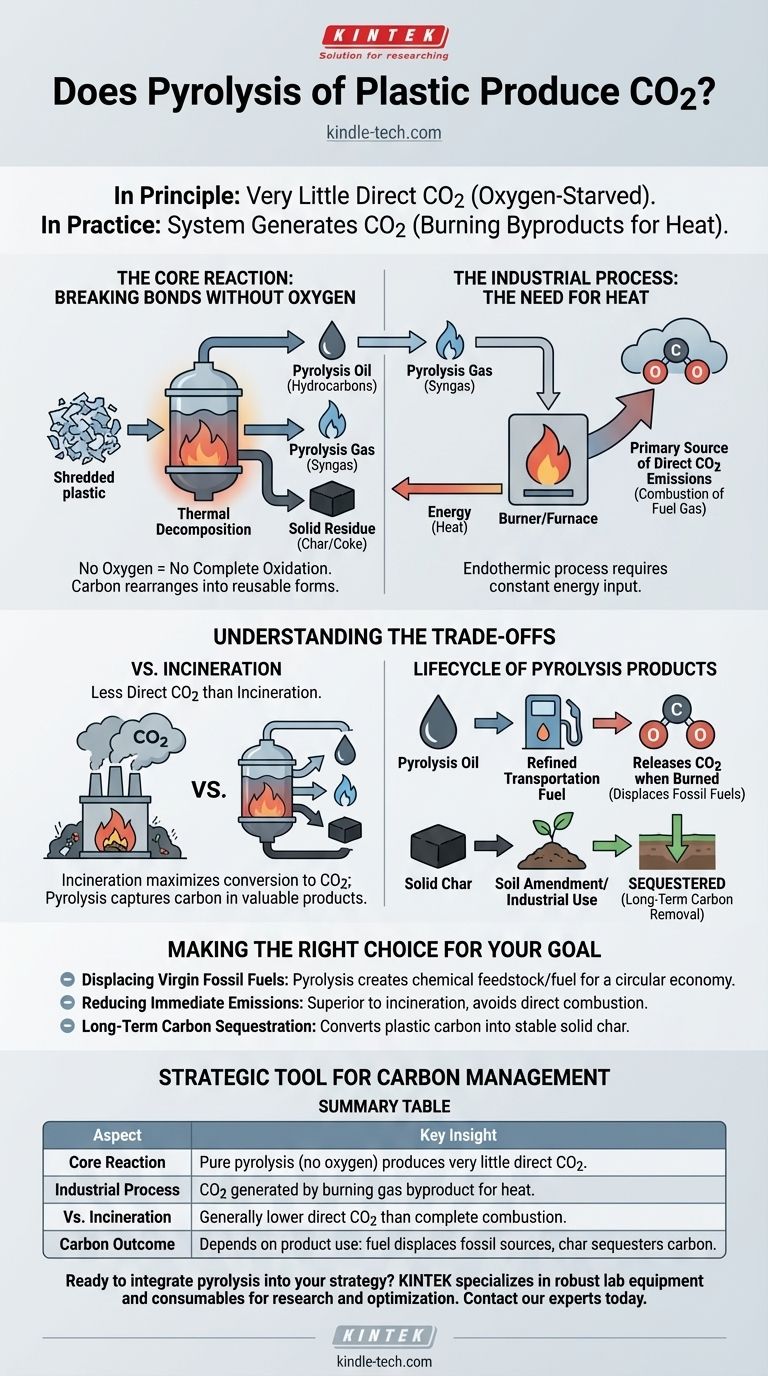
In principle, pure plastic pyrolysis produces very little direct CO2. This is because the process involves heating plastic waste in an environment with little to no oxygen. However, in practice, a complete plastic pyrolysis system does generate CO2, primarily from the combustion of its own gaseous byproducts to fuel the reaction.
The critical distinction is between the core chemical reaction and the industrial plant as a whole. While the oxygen-starved pyrolysis reaction itself minimizes CO2 formation, the energy required to run the process is typically generated by burning byproducts, which is the main source of CO2 emissions.

The Reaction vs. The Plant: Two Sources of Emissions
Understanding the environmental profile of plastic pyrolysis requires separating the core chemical change from the engineering realities of an operational facility.
The Core Reaction: Breaking Bonds Without Oxygen
Pyrolysis is a process of thermal decomposition. Instead of burning the plastic (which requires oxygen), it uses intense heat to break the long polymer chains into smaller, more valuable molecules.
Without sufficient oxygen, the carbon atoms in the plastic cannot fully oxidize to form carbon dioxide (CO2). Instead, they rearrange to form three primary products:
- Pyrolysis Oil: A liquid mixture of various hydrocarbons, similar to crude oil.
- Pyrolysis Gas (Syngas): A mix of flammable gases like hydrogen, methane, and carbon monoxide.
- Solid Residue (Char/Coke): A solid, carbon-rich material.
The Industrial Process: The Need for Heat
The pyrolysis reaction is endothermic, meaning it requires a constant input of significant energy to maintain the high temperatures needed to break down the plastic.
The most economically viable way to supply this heat is to use a portion of the pyrolysis gas produced during the process. This gas is piped back to a burner or furnace to heat the main reactor. When this fuel gas is burned, its carbon-containing components (like methane and carbon monoxide) react with oxygen from the air, releasing their energy and producing CO2. This is the primary source of direct CO2 emissions from a pyrolysis plant.
Understanding the Trade-offs
No technology is a perfect solution. The value of pyrolysis lies in how it compares to the alternatives and how its products are used.
Pyrolysis vs. Incineration
Compared to incineration (burning plastic for energy), pyrolysis generally releases less CO2 at the plant. Incineration's entire purpose is the complete combustion of waste in an oxygen-rich environment, which maximizes the immediate conversion of the plastic's carbon into CO2. Pyrolysis, by contrast, aims to capture that carbon in the form of a reusable oil or a stable solid char.
The Lifecycle of Pyrolysis Products
The ultimate carbon footprint depends on what happens to the final products.
If the pyrolysis oil is refined and used as a transportation fuel, the carbon it contains will be released as CO2 when that fuel is eventually burned. The benefit, however, is the displacement of fossil fuels that would have otherwise been extracted from the ground.
If the solid char is used as a soil amendment (biochar) or for other industrial purposes, that carbon is effectively sequestered, keeping it out of the atmosphere for long periods. This represents a true carbon removal pathway.
Making the Right Choice for Your Goal
Evaluating plastic pyrolysis requires clarity on the intended environmental outcome.
- If your primary focus is displacing virgin fossil fuels: Pyrolysis is a powerful tool for converting plastic waste into a chemical feedstock or fuel, creating a more circular economy.
- If your primary focus is reducing immediate emissions from waste management: Pyrolysis is typically superior to incineration because it avoids the direct, complete combustion of plastic into atmospheric CO2.
- If your primary focus is long-term carbon sequestration: The process offers a unique benefit by converting a significant portion of the plastic's carbon into a stable, solid char that can be stored.
Ultimately, viewing plastic pyrolysis not as a zero-emission panacea, but as a strategic tool for carbon management and resource recovery, provides the clearest path forward.
Summary Table:
| Aspect | Key Insight |
|---|---|
| Core Reaction | Pure pyrolysis (no oxygen) produces very little direct CO2 by breaking plastic into oil, gas, and char. |
| Industrial Process | CO2 is generated by burning the process's own gas byproduct to fuel the energy-intensive reaction. |
| Vs. Incineration | Pyrolysis avoids the complete, immediate combustion of plastic, generally resulting in lower direct CO2 emissions. |
| Carbon Outcome | Depends on product use: oil displaces fossil fuels; stable char can sequester carbon long-term. |
Ready to integrate pyrolysis into your waste management or resource recovery strategy?
At KINTEK, we specialize in providing robust lab equipment and consumables to help you research, develop, and optimize pyrolysis processes. Whether you are a researcher focused on carbon sequestration or an engineer developing a circular economy solution, our tools can support your goals for efficient and sustainable plastic waste management.
Contact our experts today to discuss how KINTEK can equip your laboratory for success.
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
People Also Ask
- At what temperature is conventional pyrolysis done? Unlock the Right Temperature for Your Desired Product
- What temperature is needed for pyrolysis waste? A Guide to Optimizing Your Waste-to-Value Process
- How is a high-temperature calcination furnace utilized in BZY20 Sol-gel? Achieve Pure Cubic Perovskite Phases
- What is the temperature of a rotary hearth furnace? Find the Right Heat for Your Process
- What is the difference between pyrolysis combustion and gasification? A Guide to Thermal Conversion Technologies



















