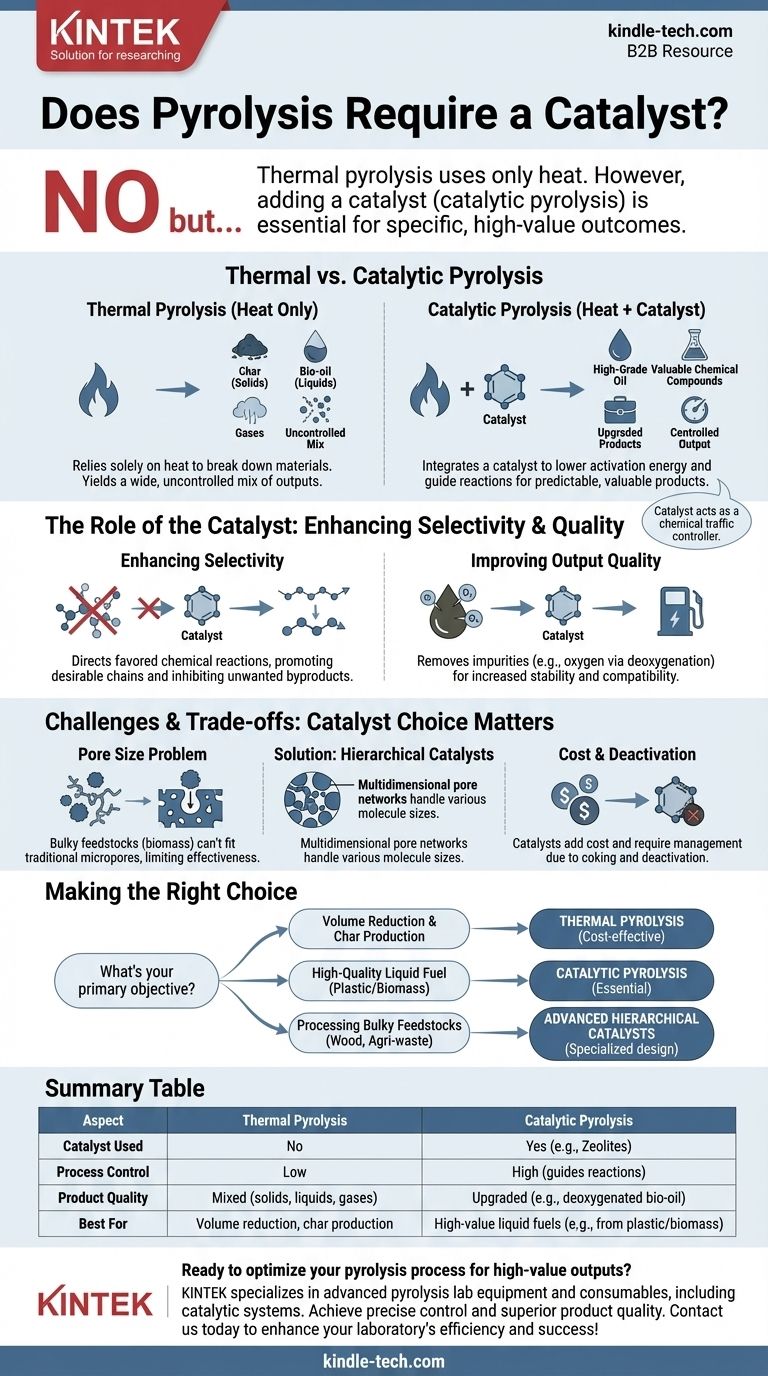
No, pyrolysis does not strictly require a catalyst, but using one is often essential for achieving specific, high-value outcomes. The base process, known as thermal pyrolysis, simply uses heat to decompose material in an oxygen-free environment. However, adding a catalyst—a process called catalytic pyrolysis—is a critical tool for guiding the chemical reactions to produce higher quality, more desirable products like transportation-grade fuels from waste plastic or biomass.
While pyrolysis can occur with heat alone, the real challenge is controlling the output. A catalyst is the key instrument that gives operators precise control, allowing them to selectively produce valuable chemical compounds and upgrade low-quality feedstock into high-grade oil.

The Fundamental Role of Catalysts in Pyrolysis
Pyrolysis is, at its core, the thermal decomposition of organic material at elevated temperatures without oxygen. The introduction of a catalyst fundamentally changes the process and its results.
Thermal vs. Catalytic Pyrolysis
Thermal pyrolysis relies solely on heat to break down complex organic polymers into smaller, simpler molecules. This process often yields a wide, uncontrolled mix of solids (char), liquids (bio-oil), and gases.
Catalytic pyrolysis integrates a catalyst into the process. The catalyst provides an active surface that lowers the activation energy required for specific chemical reactions, guiding the decomposition pathways toward a more predictable and valuable output.
Enhancing Reaction Selectivity
The primary function of a catalyst is to direct, or select, which chemical reactions are favored. Without a catalyst, the breakdown of materials like plastic or biomass is chaotic.
A catalyst acts like a chemical traffic controller, promoting reactions that create desirable hydrocarbon chains (like those in gasoline or diesel) while inhibiting the formation of unwanted byproducts.
Improving Output Quality
For applications like converting biomass or plastic into liquid fuel, the quality of the resulting oil is paramount. Catalysts play a crucial role here.
For example, in biomass pyrolysis, catalysts help remove oxygen from the bio-oil (a process called deoxygenation), which increases its energy density and stability, making it more compatible with conventional fuels.
Common Catalysts and Their Challenges
The choice of catalyst depends heavily on the feedstock and the desired product. Commercial catalysts are effective but come with their own set of limitations.
Zeolite-Based Catalysts
Zeolites are crystalline aluminosilicates with a highly ordered, porous structure. They are the most common type of catalyst used in both petrochemical refining and catalytic pyrolysis.
Their well-defined pores and acidic sites are highly effective at "cracking" long hydrocarbon chains into smaller, more valuable molecules, making them ideal for converting waste plastics into oil.
The Challenge of Bulky Feedstocks
A significant challenge arises when using conventional catalysts for biomass pyrolysis. Natural polymers found in biomass, such as cellulose and lignin, are much larger and bulkier than the molecules found in crude oil.
The narrow micropores of traditional zeolites are often too small for these large biomass-derived molecules to enter. This "molecular traffic jam" severely limits the catalyst's effectiveness.
Understanding the Trade-offs
While catalysts offer significant advantages, they also introduce complexity and cost. Understanding these trade-offs is essential for designing an efficient and economical process.
The Pore Size Problem
As noted, the pore structure of the catalyst is critical. If the pores are too small for the feedstock molecules, the catalytic process will fail.
The solution is to design hierarchical catalysts. These advanced materials feature a multidimensional network of pores—ranging from large macropores and medium mesopores to small micropores. This structure allows large molecules to enter and be broken down before reaching the smaller, highly active sites.
Catalyst Cost and Deactivation
Catalysts are a significant operational expense. They are often made from specialized, costly materials.
Furthermore, catalysts can become deactivated over time due to coking, where carbon deposits accumulate on the active surfaces and block the pores. This requires a regeneration step (burning off the coke) or eventual replacement, adding to the process complexity and cost.
Making the Right Choice for Your Goal
The decision to use a catalyst depends entirely on your objective. There is no single "best" approach; the optimal process is defined by the desired outcome.
- If your primary focus is simple volume reduction or producing char: Thermal pyrolysis is often sufficient and more cost-effective.
- If your primary focus is producing high-quality liquid fuel from plastic or biomass: Catalytic pyrolysis is essential to control the product distribution and upgrade the oil quality.
- If your primary focus is processing bulky feedstocks like wood or agricultural waste: You must consider advanced hierarchical catalysts designed to handle large molecules effectively.
Ultimately, choosing the right pyrolysis pathway is an engineering decision that balances product value against operational complexity and cost.
Summary Table:
| Aspect | Thermal Pyrolysis | Catalytic Pyrolysis |
|---|---|---|
| Catalyst Used | No | Yes (e.g., Zeolites) |
| Process Control | Low | High (guides reactions) |
| Product Quality | Mixed (solids, liquids, gases) | Upgraded (e.g., deoxygenated bio-oil) |
| Best For | Volume reduction, char production | High-value liquid fuels (e.g., from plastic/biomass) |
Ready to optimize your pyrolysis process for high-value outputs? KINTEK specializes in lab equipment and consumables for advanced pyrolysis applications, including catalytic systems. Whether you're converting waste plastic into fuel or upgrading biomass, our solutions help you achieve precise control and superior product quality. Contact us today to discuss how our expertise can enhance your laboratory's efficiency and success!
Visual Guide
Related Products
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- What are the zones in rotary kiln in cement production? Master the Core Process for High-Quality Clinker
- What is the purpose of a calciner? Boost Efficiency in High-Temperature Processing
- What are the industrial applications of pyrolysis? Transform Waste into Energy and Valuable Products
- What is the difference between calcining and roasting? A Guide to High-Temperature Processing
- What equipment is used in pyrolysis? Choosing the Right Reactor for Your Feedstock and Products



















