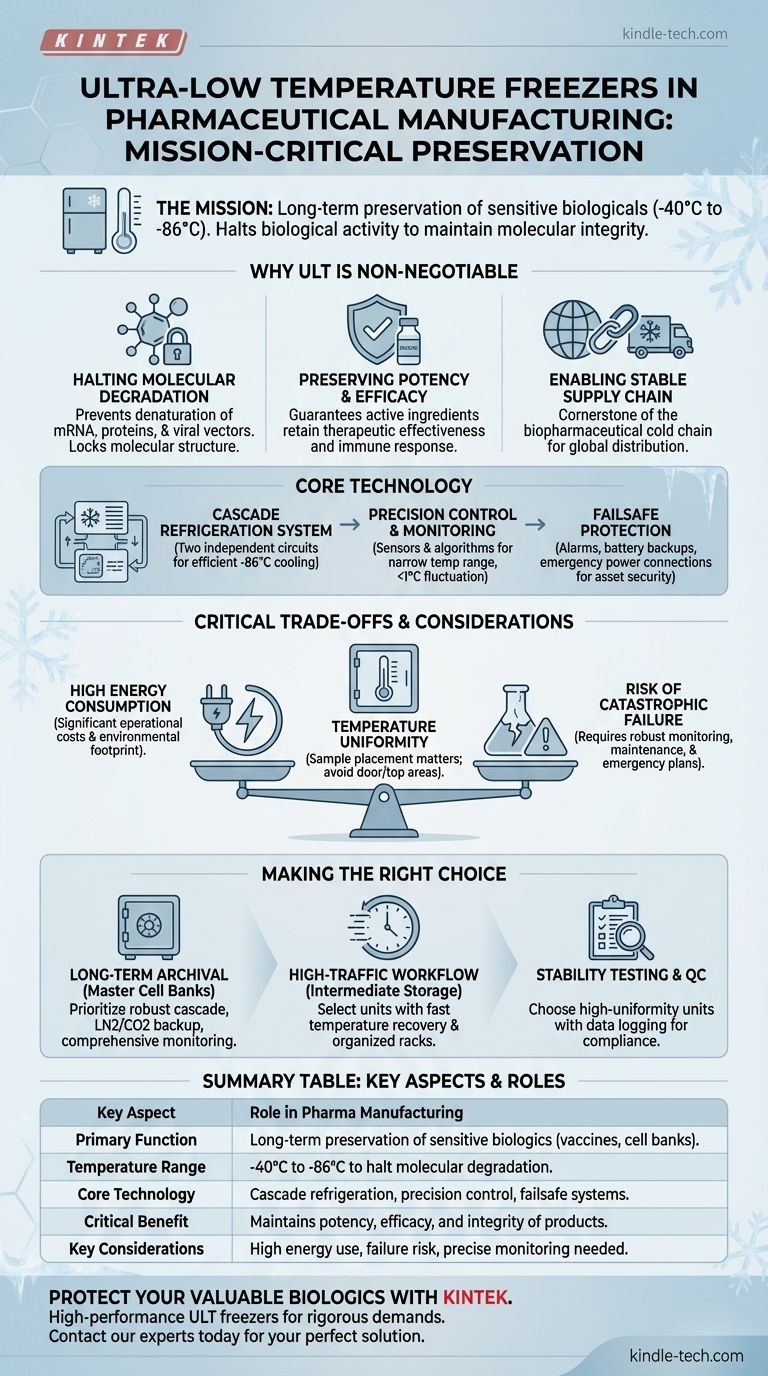
In pharmaceutical manufacturing, ultra-low temperature (ULT) freezers are mission-critical instruments used for the long-term preservation of highly sensitive biological materials. By maintaining temperatures between -40°C and -86°C, they effectively halt biological activity, ensuring that assets like vaccines, cell cultures, and complex biopharmaceuticals retain their molecular integrity, potency, and therapeutic efficacy from production through to storage.
A ULT freezer is more than just cold storage; it is a precision-engineered environment that creates a state of suspended animation for biologics. Understanding its function is to understand how the value and viability of modern medicines are protected against degradation and time.

Why Ultra-Low Temperatures Are Non-Negotiable
The fundamental purpose of a ULT freezer is to stop the clock on biological degradation. At standard refrigeration or even conventional freezer temperatures, complex molecules remain susceptible to processes that can render them ineffective or unsafe.
Halting Molecular Degradation
Many modern pharmaceuticals, including vaccines and gene therapies, are composed of delicate molecules like mRNA, proteins, and viral vectors.
At warmer temperatures, these molecules can denature, meaning they lose their specific three-dimensional shape. This structural change is often irreversible and results in a complete loss of function.
ULT environments effectively lock these molecules in place, preventing the molecular motion that leads to denaturation and preserving their intended structure.
Preserving Potency and Efficacy
For biologics, potency is directly tied to the integrity of the active ingredients. A vaccine with damaged mRNA or denatured proteins will not trigger the correct immune response.
By maintaining materials in a deep-frozen state, manufacturers guarantee that the product's potency remains consistent over long periods. This is essential for both regulatory compliance and patient safety.
Enabling a Stable Supply Chain
ULT freezers are a cornerstone of the biopharmaceutical cold chain. They allow manufacturers to create and stockpile large batches of products like cell banks, enzymes, or vaccines.
This stability enables global distribution and ensures that a ready supply of critical medicine is available, a practice that became highly visible during the distribution of SARS-CoV-2 vaccines.
The Core Technology of a ULT Freezer
A ULT freezer is not simply a more powerful version of a household unit. It relies on specialized engineering to achieve and maintain extreme temperatures reliably.
The Cascade Refrigeration System
Many ULT freezers use a cascade system involving two independent refrigeration circuits. The first circuit cools the condenser of the second, allowing the second circuit to achieve much lower temperatures than a single compressor could.
This two-stage process is highly efficient at removing heat and is fundamental to reaching the -80°C range.
Precision Temperature Control and Monitoring
Maintaining a precise temperature is critical. ULT freezers use a network of sensors and control algorithms to constantly monitor the internal environment.
This system creates a feedback loop that adjusts the cooling cycle to hold the temperature within a very narrow range of the setpoint, often with less than a degree of fluctuation.
Failsafes for Asset Protection
Given the high value of the materials stored, reliability is paramount. These freezers are equipped with multiple failsafe systems.
This includes on-board alarms for temperature deviations, door-ajar alerts, and connections for external monitoring. Crucially, they often incorporate battery backups for the control panel or connections for emergency power (like a generator) to protect billions of dollars in assets during a power outage.
Understanding the Critical Trade-offs
While essential, deploying ULT freezers requires careful planning and an awareness of their operational demands and risks.
Significant Energy Consumption
Achieving and maintaining ultra-low temperatures is an energy-intensive process. These units are among the most power-hungry pieces of equipment in a laboratory or manufacturing suite.
This results in high operational costs and a considerable environmental footprint that facilities must manage.
The Risk of Catastrophic Failure
A freezer failure is not an inconvenience; it can be a catastrophic event, leading to the complete loss of irreplaceable cell lines or millions of dollars worth of product.
This risk is why robust monitoring, regular maintenance, and a well-rehearsed emergency response plan are non-negotiable components of their use.
Temperature Uniformity vs. Setpoint
The temperature displayed on the front of the freezer is the setpoint, but the temperature inside is not perfectly uniform. Areas near the door or at the top of the unit may be slightly warmer.
Proper inventory management and sample placement are critical to ensure the most sensitive materials are stored in the most stable locations, typically in the center and back of the chamber.
Making the Right Choice for Your Goal
The application of a ULT freezer should align with the specific material being stored and its role in your process.
- If your primary focus is long-term archival of master cell banks: Prioritize freezers with the most robust cascade systems, liquid nitrogen (LN2) or CO2 backup systems, and comprehensive monitoring.
- If your primary focus is intermediate storage in a high-traffic workflow: Select units known for fast temperature recovery after door openings and invest in efficient, well-organized rack systems to minimize search time.
- If your primary focus is stability testing and quality control: Choose a smaller, high-performance unit that can guarantee exceptional temperature uniformity and data logging for regulatory compliance.
Ultimately, mastering the use of ultra-low temperature technology is fundamental to protecting the integrity and value of modern biological assets.
Summary Table:
| Key Aspect | Role in Pharmaceutical Manufacturing |
|---|---|
| Primary Function | Long-term preservation of sensitive biologics (vaccines, cell banks) |
| Temperature Range | -40°C to -86°C to halt molecular degradation |
| Core Technology | Cascade refrigeration systems for reliable ultra-low temps |
| Critical Benefit | Maintains potency, efficacy, and integrity of therapeutic products |
| Key Considerations | High energy use, risk of catastrophic failure, need for precise monitoring |
Protect your most valuable biologics with confidence. KINTEK specializes in high-performance lab equipment, including ultra-low temperature freezers designed for the rigorous demands of pharmaceutical manufacturing. Our solutions ensure your vaccines, cell cultures, and biopharmaceuticals maintain their integrity from production to storage.
Contact our experts today to find the perfect ULT freezer for your laboratory's specific needs and safeguard your critical assets.
Visual Guide
Related Products
- 58L Precision Laboratory Ultra Low Temperature Upright Freezer for Critical Sample Storage
- 28L Compact Upright Ultra Low Temperature Freezer for Laboratory
- 158L Precision Vertical Ultra Low Freezer for Laboratory Applications
- 808L Precision Laboratory Vertical Ultra Low Temperature Freezer
- 108L Vertical Ultra Low Temperature ULT Freezer
People Also Ask
- What are ultra-low temperature freezers designed for? Preserving Your Most Valuable Biological Samples
- How do ultra-low temperature freezers work? Unlocking the Secrets of -86°C Sample Preservation
- How do ultra-low temperature freezers achieve such low temperatures? The Science Behind -80°C Cooling
- What features do ultra-low temperature freezers typically include? Ensuring Absolute Sample Security
- What is the temperature control capability of ultra-low freezers? Precise Stability Down to -86°C



















