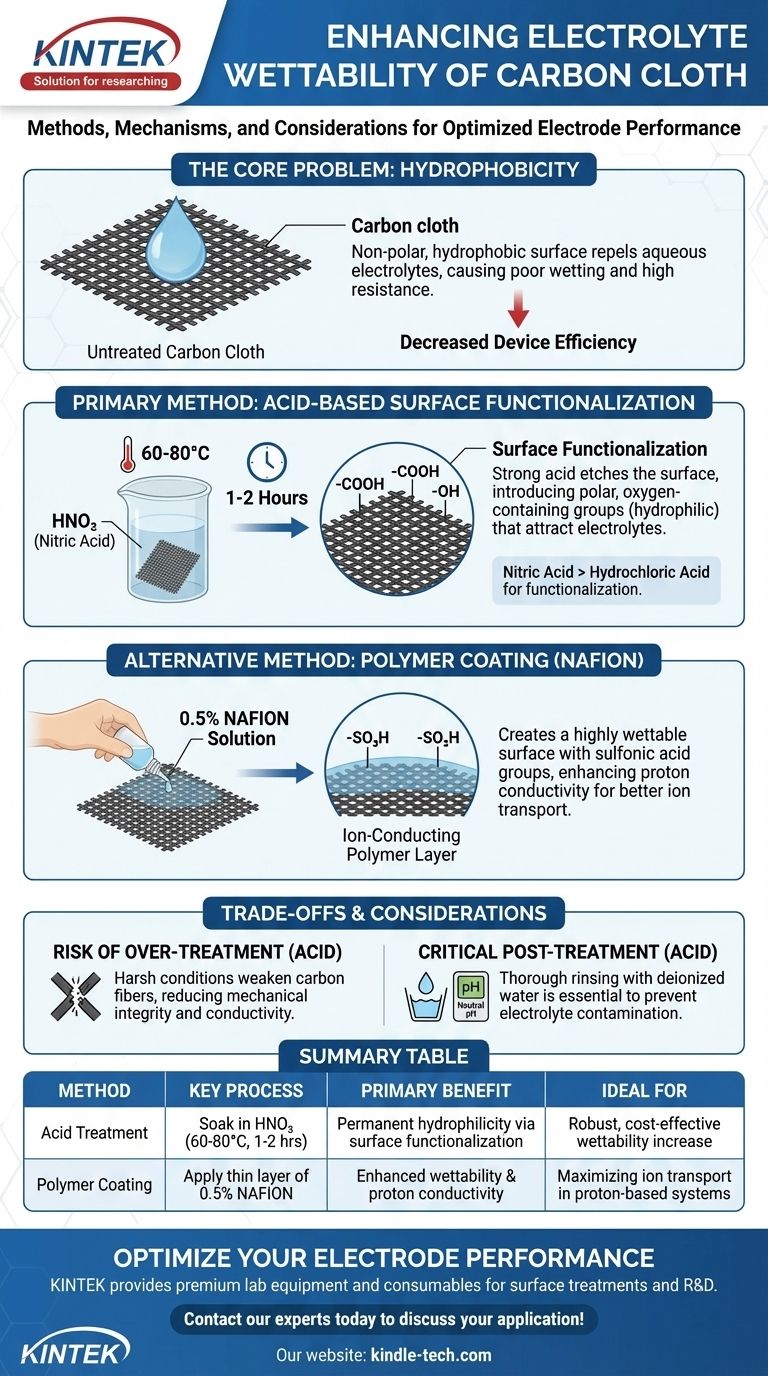
To directly enhance the electrolyte wettability of carbon cloth, you can perform a chemical pretreatment. The most common and effective method is to soak the material in an oxidizing acid, such as nitric acid (HNO₃), at an elevated temperature of 60-80°C for 1 to 2 hours. This process fundamentally alters the surface chemistry of the carbon fibers, making them more receptive to aqueous electrolytes.
The core challenge with carbon cloth is its inherently non-polar, hydrophobic surface, which repels polar electrolytes. The solution is not merely to clean the surface, but to chemically engineer it by introducing polar, oxygen-containing functional groups that attract the electrolyte.

The Core Problem: Hydrophobicity of Carbon Cloth
Why Untreated Carbon Resists Electrolytes
Untreated carbon cloth is primarily composed of graphitic carbon. Its surface is non-polar and lacks the chemical affinity to bond with polar molecules, such as the water found in most aqueous electrolytes.
This property, known as hydrophobicity, causes the electrolyte to bead up on the surface rather than wicking into the intricate fibrous structure of the cloth.
The Impact on Device Performance
This poor wetting has severe consequences for any electrochemical device. It creates a high interfacial resistance between the electrode and the electrolyte, impeding the flow of ions.
Furthermore, a significant portion of the electrode's potential surface area remains unused, drastically reducing the device's overall efficiency, power density, and capacity.
Primary Method: Acid-Based Surface Functionalization
The Mechanism of Acid Treatment
The most reliable way to improve wettability is through surface functionalization using an oxidizing acid. Strong acids, particularly nitric acid (HNO₃), react with the carbon surface at elevated temperatures.
This reaction etches the surface on a microscopic level and, more importantly, introduces polar, oxygen-containing functional groups such as carboxyl (-COOH) and hydroxyl (-OH) groups. These groups are hydrophilic (water-loving) and act as anchor points for the polar electrolyte.
Recommended Process Parameters
Based on established procedures, a typical treatment involves immersing the carbon cloth in nitric acid or hydrochloric acid.
The key parameters are a temperature of 60-80°C and a duration of 1-2 hours. This combination provides enough thermal energy to activate the chemical reaction without causing excessive structural damage to the carbon fibers.
Nitric Acid vs. Hydrochloric Acid
While both acids are mentioned, they serve slightly different functions. Nitric acid is a powerful oxidizing agent and is far more effective at creating the desired oxygen functional groups.
Hydrochloric acid (HCl) is not an oxidizing agent. Its primary role is to clean the surface of impurities, though it can induce some minor changes. For enhancing wettability, nitric acid is the superior choice.
Alternative Method: Polymer Coating with NAFION
How NAFION Enhances Wettability
An alternative approach is to coat the carbon fibers with a thin layer of an ion-conducting polymer, such as NAFION.
NAFION contains sulfonic acid (-SO₃H) groups, which are extremely hydrophilic. A 0.5% NAFION solution can be used to coat the fibers, creating a new, highly wettable surface that readily draws in the electrolyte.
Dual Benefits: Wettability and Ion Conduction
The advantage of a NAFION coating extends beyond simple wettability. As an ionomer, NAFION actively facilitates the transport of ions (specifically protons) through the electrode structure.
This creates a highly conductive pathway for ions, further reducing internal resistance and improving overall device performance, which is especially critical in fuel cells and certain flow batteries.
Understanding the Trade-offs and Considerations
Risk of Over-Treatment with Acid
While effective, acid treatment is a destructive process. If the conditions are too harsh—either by using too high a concentration, temperature, or duration—it can weaken the carbon fibers.
This can lead to reduced mechanical integrity and a loss of electrical conductivity, negatively impacting the long-term stability of the electrode. Careful control is essential.
Post-Treatment Rinsing is Crucial
After acid treatment, it is absolutely critical to rinse the carbon cloth thoroughly with deionized water. This must be done until the rinse water reaches a neutral pH.
Failure to remove all residual acid will contaminate your electrolyte, leading to side reactions, corrosion, and rapid degradation of your electrochemical cell.
Making the Right Choice for Your Application
Choosing the correct method depends on your specific goals and the system you are building.
- If your primary focus is a robust, cost-effective increase in hydrophilicity: Nitric acid treatment is the standard and most direct method for permanently modifying the carbon surface.
- If your primary focus is maximizing ion transport in a proton-based system: A NAFION coating offers the dual benefit of excellent wettability and enhanced proton conductivity.
- If you are concerned about preserving mechanical strength: Begin your acid treatment process with milder conditions (e.g., 60°C for 1 hour) and test the results before moving to more aggressive treatments.
Ultimately, mastering the surface chemistry of your electrode is the key to unlocking the full performance potential of your electrochemical device.
Summary Table:
| Method | Key Process | Primary Benefit | Ideal For |
|---|---|---|---|
| Acid Treatment | Soak in HNO₃ (60-80°C, 1-2 hrs) | Permanent hydrophilicity via surface functionalization | Robust, cost-effective wettability increase |
| Polymer Coating | Apply thin layer of 0.5% NAFION | Enhanced wettability & proton conductivity | Maximizing ion transport in proton-based systems |
Ready to Optimize Your Electrode Performance?
Unlocking the full potential of your electrochemical devices starts with the right materials and expertise. KINTEK specializes in premium lab equipment and consumables, providing the reliable tools and support you need to perfect surface treatments and enhance your R&D.
Let us help you achieve superior efficiency and power density. Contact our experts today to discuss your specific application needs!
Visual Guide
Related Products
- Hydrophilic Carbon Paper TGPH060 for Battery Lab Applications
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Electrolytic Electrochemical Cell for Coating Evaluation
- Isostatic Molding Pressing Molds for Lab
- Optical Window Glass Substrate Wafer CaF2 Substrate Window Lens
People Also Ask
- What is an RVC glassy carbon sheet? A High-Performance Material for Demanding Applications
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- How is carbon paper treated for use in fuel cells? The Critical PTFE Coating for Peak Performance
- What is the purpose of laminating? Protect and Enhance Your Documents for Long-Term Use
- What are the key properties of carbon felt? Unlocking High-Temperature & Electrochemical Performance









