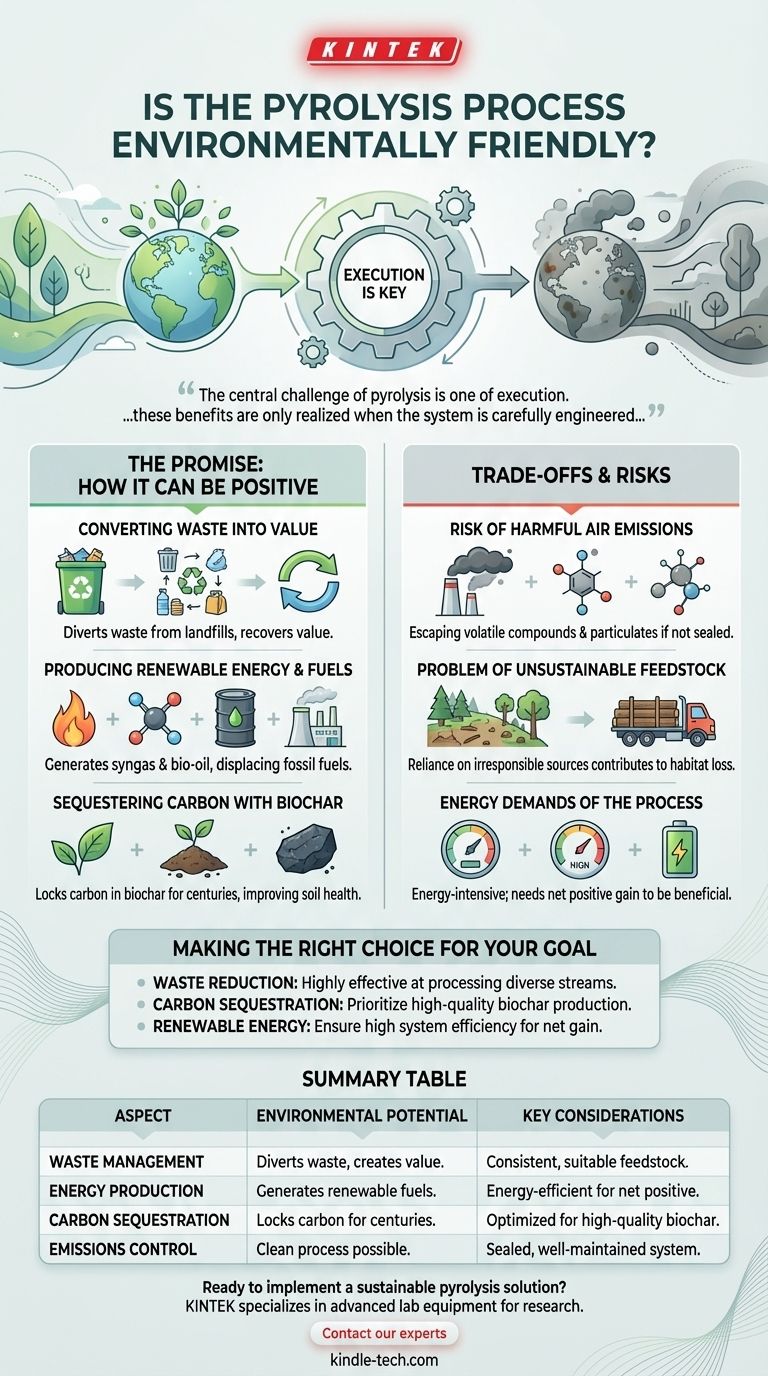
At its core, the environmental friendliness of pyrolysis is not inherent to the process itself, but is determined entirely by how it is designed, managed, and supplied. When executed correctly with sustainable inputs, it is a powerful tool for converting waste into clean energy and stable carbon. However, a poorly controlled process can create its own set of environmental problems, including air pollution and unsustainable resource use.
The central challenge of pyrolysis is one of execution. While it holds immense promise for reducing waste, creating renewable energy, and sequestering carbon, these benefits are only realized when the system is carefully engineered to control emissions and sourced with sustainable materials.

The Promise: How Pyrolysis Can Be Environmentally Positive
Pyrolysis is a thermochemical process that heats organic materials, like biomass or plastic, at high temperatures in an oxygen-free environment. This breaks them down into valuable new products instead of just burning them.
Converting Waste into Value
Pyrolysis offers a robust way to process difficult waste streams. Agricultural residues, wood scraps, and even certain municipal solid wastes can be diverted from landfills and converted into useful outputs.
This circular approach reduces the economic and environmental burden of landfilling while recovering value from materials that would otherwise be discarded.
Producing Renewable Energy and Fuels
The process generates several key products. Syngas (a mix of hydrogen and carbon monoxide) can be used to generate heat and power, while bio-oil can be refined into transportation fuels or other chemicals.
These products serve as direct replacements for fossil fuels, reducing our dependence on non-renewable resources and the pollution associated with their extraction and combustion.
Sequestering Carbon with Biochar
Perhaps the most significant environmental benefit is the creation of biochar, a stable, carbon-rich solid. Unlike burning, which releases carbon into the atmosphere, pyrolysis can lock it into this solid form.
When added to soil, biochar can remain stable for centuries, representing a form of active carbon sequestration. It also improves soil health, water retention, and agricultural productivity.
Understanding the Trade-offs and Risks
The potential benefits of pyrolysis are compelling, but they are not guaranteed. Achieving a net-positive environmental impact requires navigating significant operational challenges.
The Risk of Harmful Air Emissions
If the process is not properly sealed and managed, harmful volatile compounds and particulates can escape into the atmosphere.
Minimizing these emissions depends entirely on the quality of the furnace design, precise operational control, and diligent system maintenance. A "leaky" or inefficient system can negate many of the environmental benefits.
The Problem of Unsustainable Feedstock
The source of the organic material, or feedstock, is critical. If pyrolysis relies on biomass from irresponsibly logged forests, it can contribute to deforestation and habitat loss.
True sustainability requires using genuine waste products or biomass from sources that are managed for renewability.
The Energy Demands of the Process
Pyrolysis is an energy-intensive process that requires high temperatures. The system's overall environmental benefit hinges on its energy balance.
An efficient system will use a portion of the syngas it produces to power itself, creating a net energy gain. An inefficient one may consume more external energy than it generates, diminishing its value.
Making the Right Choice for Your Goal
To assess if pyrolysis is the right solution, you must first define your primary environmental objective.
- If your primary focus is waste reduction: Pyrolysis is highly effective at processing diverse organic waste streams, significantly reducing landfill volume and creating valuable byproducts.
- If your primary focus is carbon sequestration: Prioritize a process optimized for high-quality biochar production, as this is the most direct pathway to long-term carbon removal.
- If your primary focus is renewable energy: Ensure the system is highly efficient, designed to maximize the output of syngas and bio-oil while powering its own operation.
Ultimately, the environmental success of a pyrolysis project is a direct result of responsible engineering, sustainable sourcing, and meticulous operational management.
Summary Table:
| Aspect | Environmental Potential | Key Considerations |
|---|---|---|
| Waste Management | Diverts waste from landfills; converts it into valuable products. | Requires consistent, suitable feedstock supply. |
| Energy Production | Generates renewable syngas and bio-oil, displacing fossil fuels. | System must be energy-efficient for a net positive gain. |
| Carbon Sequestration | Produces stable biochar that locks carbon in soil for centuries. | Process must be optimized for high-quality biochar yield. |
| Emissions Control | Can be a clean process with no direct combustion emissions. | Dependent on sealed, well-maintained, and controlled systems. |
Ready to implement a sustainable pyrolysis solution for your laboratory or operation?
KINTEK specializes in advanced lab equipment and consumables for pyrolysis research and development. Our reliable systems help you achieve precise temperature control, maximize product yield, and ensure operational safety. Whether your goal is waste valorization, biochar production, or renewable energy studies, we provide the technology and support to make your project environmentally and economically successful.
Contact our experts today to discuss how our pyrolysis solutions can meet your specific sustainability goals.
Visual Guide
Related Products
- Graphite Vacuum Continuous Graphitization Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Vertical Laboratory Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- Is graphite affected by heat? Discover Its Remarkable Strength and Stability at High Temperatures
- What is the temperature resistance of graphite? Unlocking Its High-Temp Potential in Your Lab
- Is a graphite melting point high or low? Discover Its Extreme Thermal Resilience
- Is graphite good in high temperature? Unlocking Its Extreme Heat Potential
- What is the thermal limit of graphite? Unlock Extreme Heat Performance in Your Lab



















