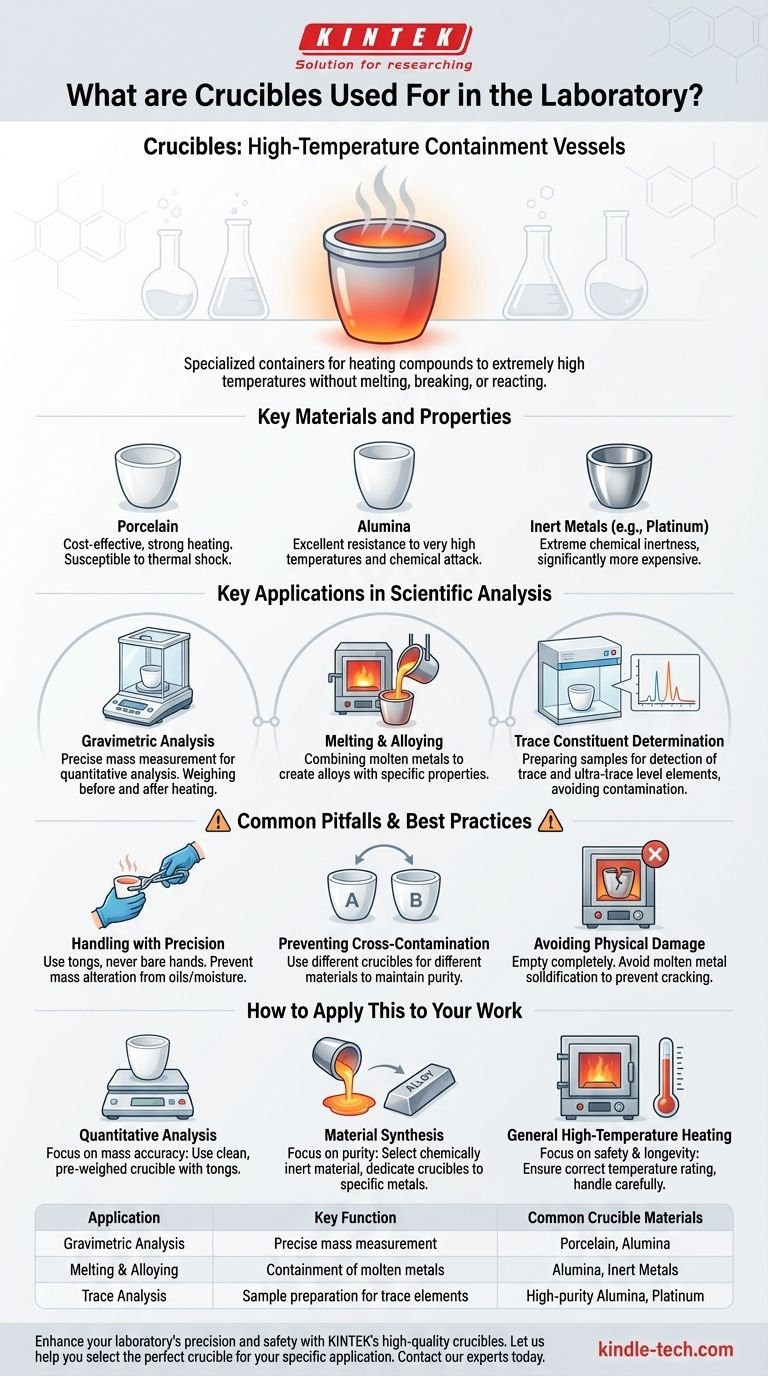
In a laboratory, crucibles are specialized containers designed to hold chemical compounds when they need to be heated to extremely high temperatures. Their primary purpose is to provide a stable, chemically inert vessel that can withstand intense heat without melting, breaking, or reacting with the substance inside. This makes them essential for processes like melting metals, creating alloys, and performing high-precision chemical analysis.
A crucible is not just a heat-proof cup; it is a critical instrument for quantitative analysis and material synthesis, where thermal stability and chemical inertness are paramount to achieving accurate results.

The Core Function: High-Temperature Containment
A crucible's value comes from its ability to safely contain materials while they undergo extreme thermal processing. This is a foundational requirement for many scientific and industrial procedures.
What is a Crucible?
At its simplest, a crucible is a cup-shaped piece of laboratory equipment. Unlike a beaker or flask, it is not made of glass but of materials specifically chosen for their high melting points and resistance to thermal shock.
Key Materials and Their Properties
Crucibles are typically made from ceramic or metallic materials that remain stable at high temperatures.
- Porcelain: A common and cost-effective choice, suitable for strong heating but can be susceptible to thermal shock if heated or cooled too rapidly.
- Alumina: A high-purity ceramic that offers excellent resistance to very high temperatures and chemical attack.
- Inert Metals: Materials like platinum are used when extreme chemical inertness is required, though they are significantly more expensive.
Key Applications in Scientific Analysis
While the function is simple containment, the applications are precise and varied, particularly in the field of analytical chemistry.
Gravimetric Analysis
This is a classic and highly accurate analytical technique where crucibles are essential. The process involves precisely measuring mass to determine the quantity of a substance.
A clean crucible is first heated to a high temperature to burn off any moisture or impurities, cooled in a desiccator, and then weighed with extreme accuracy. The sample is added, and the crucible is heated again to induce a chemical change, such as driving off volatile components. After cooling, it is weighed again, and the difference in mass reveals the mass of the remaining residue.
Melting and Alloying
In metallurgy and materials science, crucibles are used to melt pure metals or metallic powders. By combining different molten metals within the crucible, scientists and engineers can create new alloys with specific, desired properties.
Trace Constituent Determination
For highly sensitive analytical work, crucibles are used to prepare samples for the detection of trace and ultra-trace level elements. The inert nature of the crucible ensures that the sample is not contaminated before analysis.
Common Pitfalls and Best Practices
A crucible is a durable tool, but improper handling can compromise experimental results and lead to damage.
Handling with Precision
Crucibles should never be handled with bare hands, as oils and moisture can alter their mass and affect results. They must be moved using properly fitting tongs, especially when hot, to ensure a secure grip and prevent accidents.
Preventing Cross-Contamination
To maintain the purity of reactions, it is critical to use different crucibles for different materials, especially when working with various metals. This practice prevents the contamination of a new sample with residue from a previous one.
Avoiding Physical Damage
After use, a crucible should be completely emptied. If molten metal is left to solidify inside, it can expand upon reheating and crack the crucible. To prevent the crucible from fusing to the furnace floor, a protective layer, such as a piece of cardboard, can be placed underneath it before heating.
How to Apply This to Your Work
The correct use of a crucible depends entirely on your scientific objective.
- If your primary focus is quantitative analysis: Your priority is mass accuracy, so use a clean, pre-weighed crucible and handle it exclusively with tongs to prevent contamination.
- If your primary focus is material synthesis: Your priority is purity, so select a crucible material that will not react with your substances and dedicate specific crucibles to specific metals to avoid cross-contamination.
- If your primary focus is general high-temperature heating: Your priority is safety and equipment longevity, so ensure your crucible is rated for the required temperature and is properly handled to avoid thermal shock or cracking.
Ultimately, a crucible is a fundamental tool that enables precise control over high-temperature chemical and physical transformations.
Summary Table:
| Application | Key Function | Common Crucible Materials |
|---|---|---|
| Gravimetric Analysis | Precise mass measurement for quantitative analysis | Porcelain, Alumina |
| Melting & Alloying | Containment of molten metals for creating alloys | Alumina, Inert Metals (e.g., Platinum) |
| Trace Analysis | Preparation of samples for detecting trace elements | High-purity Alumina, Platinum |
Enhance your laboratory's precision and safety with KINTEK's high-quality crucibles.
Whether you are conducting sensitive gravimetric analysis, synthesizing new materials, or melting metals, the right crucible is fundamental to your success. KINTEK specializes in providing durable, chemically inert lab equipment and consumables designed to withstand extreme temperatures and ensure accurate, contamination-free results.
Let us help you select the perfect crucible for your specific application. Contact our experts today to discuss your laboratory needs and discover how our solutions can improve your processes and outcomes.
Visual Guide
Related Products
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Engineering Advanced Fine Alumina Al2O3 Ceramic Crucible for Laboratory Muffle Furnace
People Also Ask
- Why is a fully closed alumina crucible required for the calcination of Wollastonite/Colemanite? Ensure Phase Purity
- What is the temperature range of graphite crucible? Choose the Right Crucible for Your High-Temp Application
- What is the primary function of a stainless steel crucible in studies involving liquid lead? Essential Lab Insights
- How do I choose a crucible? Match Material, Temperature, and Application for Success
- What is the melting point of crucible material? Choose the Right Crucible for Your Process
- Is a crucible a lab equipment? A Guide to High-Temperature Containers for Labs and Foundries
- Why are alumina crucibles used for Al-LLZ sintering? The Secret to Stable Cubic Phase Lithium Garnet
- What is a crucible used for in the crucible? The Essential Tool for Melting Metals & More



















