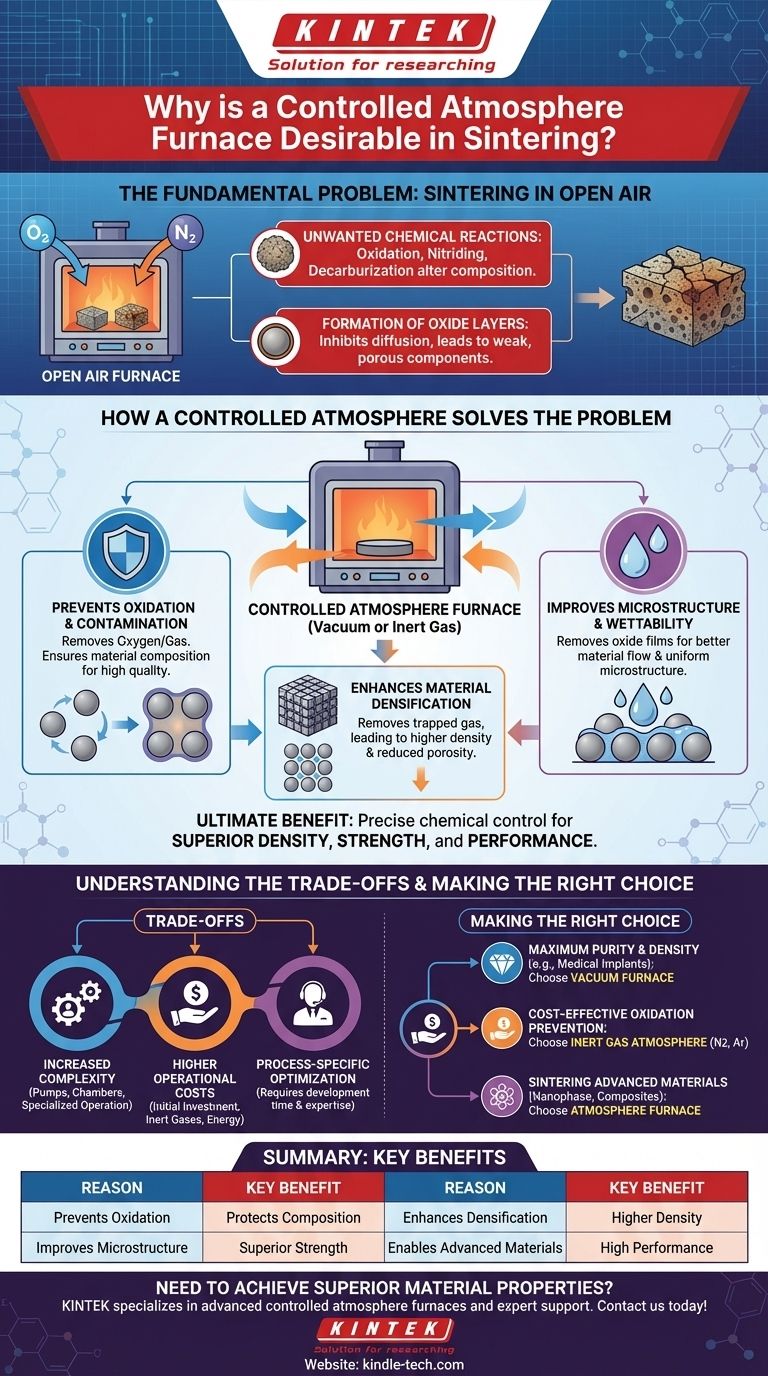
Ultimately, a controlled atmosphere furnace is desirable for sintering because it provides precise chemical control over the process. By removing or replacing reactive gases like oxygen, it prevents contamination and unwanted chemical reactions at high temperatures, which is essential for achieving superior material density, strength, and specific microstructural properties in the final product.
The core purpose of controlling the furnace atmosphere is to eliminate chemically reactive elements, primarily oxygen, that would otherwise degrade the material's integrity and performance during the high-temperature sintering cycle.

The Fundamental Problem: Sintering in Open Air
Sintering requires extremely high temperatures to fuse material particles together. When performed in an uncontrolled environment like ambient air, the process is compromised.
Unwanted Chemical Reactions
At sintering temperatures, materials are highly reactive. Oxygen and nitrogen in the air can cause detrimental chemical changes.
These reactions include oxidation, nitriding, and for certain alloys, decarburization (the loss of carbon). These changes alter the material's composition and degrade its final properties.
Formation of Oxide Layers
Even a minimal amount of oxygen can form an oxide film on the surface of the material particles.
This film can inhibit the diffusion and bonding processes that are critical for particles to fuse together, resulting in a weaker, more porous final component.
How a Controlled Atmosphere Solves the Problem
By creating a specific environment—either a vacuum or one filled with a non-reactive gas—an atmosphere furnace directly counteracts the issues of open-air sintering.
Preventing Oxidation and Contamination
The most immediate benefit is the prevention of oxidation. By removing oxygen via a vacuum or displacing it with an inert gas like argon or nitrogen, the material is chemically protected.
This ensures the sintered part maintains its intended composition, leading to a higher-quality and more predictable outcome.
Enhancing Material Densification
Sintering aims to eliminate the pores between material particles. A vacuum atmosphere is particularly effective at this.
By removing the gas trapped within these pores, the furnace makes it easier for the material to consolidate, leading to significantly higher density and reduced porosity in the final part.
Improving Microstructure and Wettability
In processes like liquid-phase sintering, a clean particle surface is essential. A controlled atmosphere removes the oxide films that can act as a barrier.
This improves the wettability of the solid particles by the liquid phase, promoting better material flow and resulting in a more uniform and robust microstructure. This directly translates to enhanced mechanical properties like strength and wear resistance.
Understanding the Trade-offs
While highly effective, controlled atmosphere sintering introduces complexities that are not present with simpler air-firing furnaces.
Increased System Complexity
These systems require vacuum pumps, sealed chambers, and sophisticated gas supply and management hardware.
This adds a layer of complexity to the equipment, requiring more specialized operation and maintenance procedures.
Higher Operational Costs
The initial investment for a controlled atmosphere furnace is higher, as are the operational costs associated with purchasing inert gases or running vacuum systems.
The process for a given material often needs to be optimized, which requires additional development time and expertise.
Process-Specific Optimization
There is no single "best" atmosphere for all materials. The ideal environment—whether vacuum, nitrogen, or argon—must be selected and fine-tuned for the specific material being sintered.
This makes the operation more involved compared to a standard furnace, demanding a higher level of process knowledge from the operator.
Making the Right Choice for Your Goal
The decision to use a controlled atmosphere depends entirely on the desired properties of the final component.
- If your primary focus is maximum purity and density: A vacuum furnace is the superior choice, as it is most effective at removing all contaminants and trapped gases, making it ideal for medical implants or rare earth magnets.
- If your primary focus is cost-effective oxidation prevention: An inert gas atmosphere (like nitrogen or argon) provides excellent protection without the higher cost and complexity of a high-vacuum system.
- If your primary focus is sintering advanced materials: Materials like nanophase composites, target materials, and functionally graded materials almost always require the precise control that only an atmosphere furnace can provide.
Controlling the furnace atmosphere is not a luxury but a critical tool for manufacturing high-performance materials that meet stringent engineering demands.
Summary Table:
| Reason | Key Benefit |
|---|---|
| Prevents Oxidation | Protects material composition by removing reactive oxygen. |
| Enhances Densification | Removes trapped gases for higher density and reduced porosity. |
| Improves Microstructure | Promotes better particle bonding for superior strength. |
| Enables Advanced Materials | Essential for sintering high-performance alloys and composites. |
Need to achieve superior material properties in your sintering process?
KINTEK specializes in providing advanced controlled atmosphere furnaces and expert support for your laboratory. Whether you are sintering advanced alloys, medical implants, or nanophase composites, our equipment ensures precise chemical control to prevent contamination and achieve maximum density and strength.
Contact us today to discuss your specific sintering requirements and discover how KINTEK's lab equipment solutions can enhance your material performance and process efficiency.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Spark Plasma Sintering Furnace SPS Furnace
People Also Ask
- Why nitrogen is used in annealing furnace? To prevent oxidation and decarburization for superior metal quality
- What provides an inert atmosphere? Achieve Safety and Purity with Nitrogen, Argon, or CO2
- What is the purpose of inert atmosphere? A Guide to Protecting Your Materials and Processes
- What gases are used in inert atmospheres? Choose the Right Gas for Non-Reactive Environments
- How we can develop inert atmosphere for a chemical reaction? Master Precise Atmospheric Control for Your Lab



















