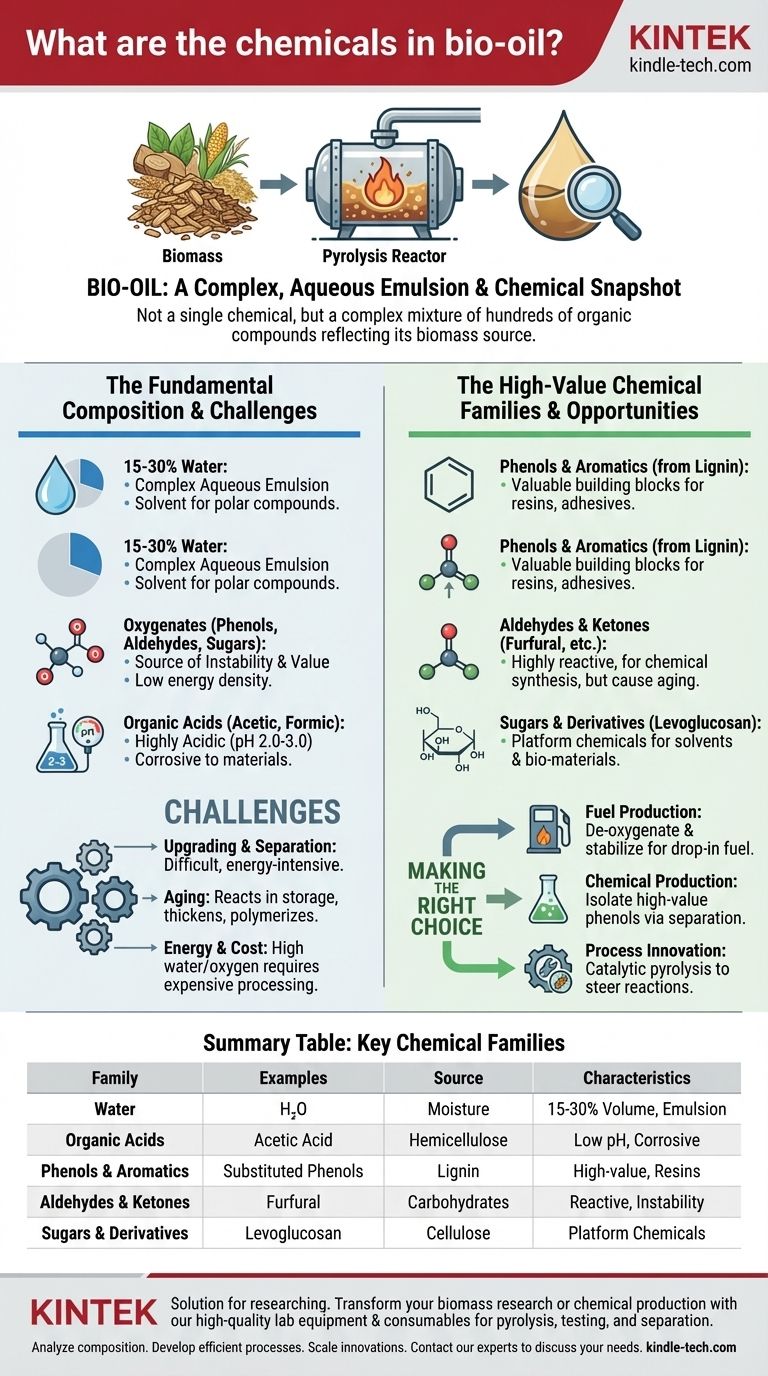
At its core, bio-oil is not a single chemical but a highly complex liquid mixture containing hundreds of distinct organic compounds. Its composition is a direct reflection of the biomass it was derived from, primarily consisting of water, organic acids, and a rich array of oxygenated compounds like phenols, aldehydes, and sugars.
Bio-oil's chemical profile presents a fundamental duality: it is a potential treasure trove of high-value specialty chemicals, particularly phenols and aromatics, but this value is locked within an unstable and corrosive mixture that is difficult and costly to refine.

The Fundamental Composition of Bio-Oil
Bio-oil is the direct liquid product of fast pyrolysis, a process that rapidly heats biomass (like wood or agricultural waste) in the absence of oxygen. Think of it as a "chemical snapshot" of the decomposed plant matter.
A Complex Aqueous Emulsion
The most significant component by mass is water, often making up 15-30% of the total volume. This water is not just mixed in; it's a solvent for many of the polar organic compounds, creating a complex, multi-phase liquid.
The Source of Instability: Oxygenates
Unlike crude petroleum, which is primarily hydrocarbons, bio-oil is rich in oxygenated organic compounds. The high oxygen content is responsible for both its potential value and its major drawbacks, such as instability and low energy density compared to fossil fuels.
Corrosive by Nature: Organic Acids
Bio-oil contains significant amounts of acetic acid and formic acid. This makes the oil highly acidic (with a pH typically between 2.0 and 3.0), rendering it corrosive to common materials like carbon steel and requiring specialized handling equipment.
The High-Value Chemical Families
The true economic potential of bio-oil lies not in its use as a raw fuel, but in the extraction of specific chemical families that serve as platform chemicals.
Phenols and Aromatics
As noted in chemical analyses, substituted phenols and other aromatics are among the most valuable components. These are derived from the breakdown of lignin in the original biomass. They are critical building blocks for producing resins, adhesives, and other specialty chemicals.
Aldehydes and Ketones
Compounds like furfural, hydroxyacetone, and formaldehyde are present in bio-oil. These are highly reactive molecules used in chemical synthesis, but they also contribute to the oil's instability, as they can polymerize over time.
Sugars and Their Derivatives
The breakdown of cellulose and hemicellulose in biomass produces sugars and anhydrosugars, most notably levoglucosan. This compound is often seen as a key platform chemical for producing solvents and other bio-based materials.
Understanding the Trade-offs: Value vs. Viability
The promise of valuable chemicals is tempered by significant technical challenges that must be overcome for bio-oil to be a viable chemical feedstock.
The Upgrading and Separation Hurdle
The hundreds of chemicals in bio-oil are all mixed together in an acidic, watery solution. Separating the valuable phenols from the low-value acids and water is the single greatest challenge. This process, known as upgrading or fractionation, is energy-intensive and expensive.
The Problem of "Aging"
Bio-oil is not a stable product. The reactive components, particularly aldehydes and acids, continue to react with each other in storage. This process, known as aging, causes the oil to thicken, form solids (polymers), and change its chemical composition, making consistent processing difficult.
The Energy and Cost Equation
Simply burning raw bio-oil for energy is inefficient due to its high water and oxygen content. Upgrading it to a stable fuel (like "green" diesel) or separating it into chemical fractions requires significant energy input, often involving hydrogen and expensive catalysts.
Making the Right Choice for Your Goal
Your approach to bio-oil's chemistry depends entirely on your end objective.
- If your primary focus is fuel production: Your goal is to de-oxygenate and stabilize the entire mixture through processes like hydrotreating to create a drop-in liquid fuel.
- If your primary focus is chemical production: Your strategy is to develop cost-effective separation techniques, like solvent extraction or fractional distillation, to isolate specific high-value chemical families like phenols.
- If your primary focus is process innovation: Your effort should be on catalytic pyrolysis, which uses catalysts during the initial production to steer the chemical reactions toward a more desirable and less complex slate of products.
Ultimately, viewing bio-oil as a renewable alternative to crude oil is to understand it as a chemically rich but challenging raw material for a future biorefinery.
Summary Table:
| Chemical Family | Key Examples | Primary Source in Biomass | Key Characteristics/Challenges |
|---|---|---|---|
| Water | H₂O | Moisture in feedstock | 15-30% of volume; creates a complex emulsion. |
| Organic Acids | Acetic Acid, Formic Acid | Hemicellulose | Causes low pH (2-3), making the oil corrosive. |
| Phenols & Aromatics | Substituted Phenols | Lignin | High-value components for resins and adhesives. |
| Aldehydes & Ketones | Furfural, Hydroxyacetone | Carbohydrates | Reactive molecules that contribute to instability ('aging'). |
| Sugars & Derivatives | Levoglucosan | Cellulose | Platform chemicals for producing bio-based materials. |
Ready to transform your biomass research or chemical production?
Understanding the complex chemistry of bio-oil is the first step. The next is having the right equipment to process, analyze, and upgrade it efficiently. KINTEK specializes in high-quality lab equipment and consumables for pyrolysis, catalytic testing, and chemical separation.
We provide the reliable tools you need to:
- Analyze bio-oil composition with precision.
- Develop efficient upgrading and separation processes.
- Scale your innovations from lab to pilot plant.
Let KINTEK be your partner in advancing renewable energy and chemicals. Contact our experts today to discuss how our solutions can meet your specific laboratory needs.
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Benchtop Laboratory Vacuum Freeze Dryer
People Also Ask
- What are the factors affecting sieving performance and efficiency? Optimize Your Particle Separation Process
- What are the specifications for test sieves? A Guide to ASTM & ISO Standards for Accurate Particle Analysis
- How is a vibratory sieve shaker used in the particle size analysis of mechanically alloyed powders? Expert Guide
- Why is sieve analysis important? Ensure Consistent Quality and Performance of Your Materials
- Why is a precision vibrating sieve shaker essential for metal leaching research? Optimize Your Particle Size Analysis
















