At their core, dental ceramics are composite materials engineered from two primary phases: a glassy, amorphous matrix and a reinforcing crystalline filler. The specific chemical components, such as silica, feldspar, alumina, and various metallic oxides, are carefully selected and proportioned to control the balance between these two phases, which ultimately dictates the material's final strength, aesthetics, and clinical application.
The essential principle to grasp is that the ratio of glass to crystals is the single most important factor in a dental ceramic's performance. More glass yields superior translucency but less strength, while more crystals provide immense strength at the cost of aesthetics.

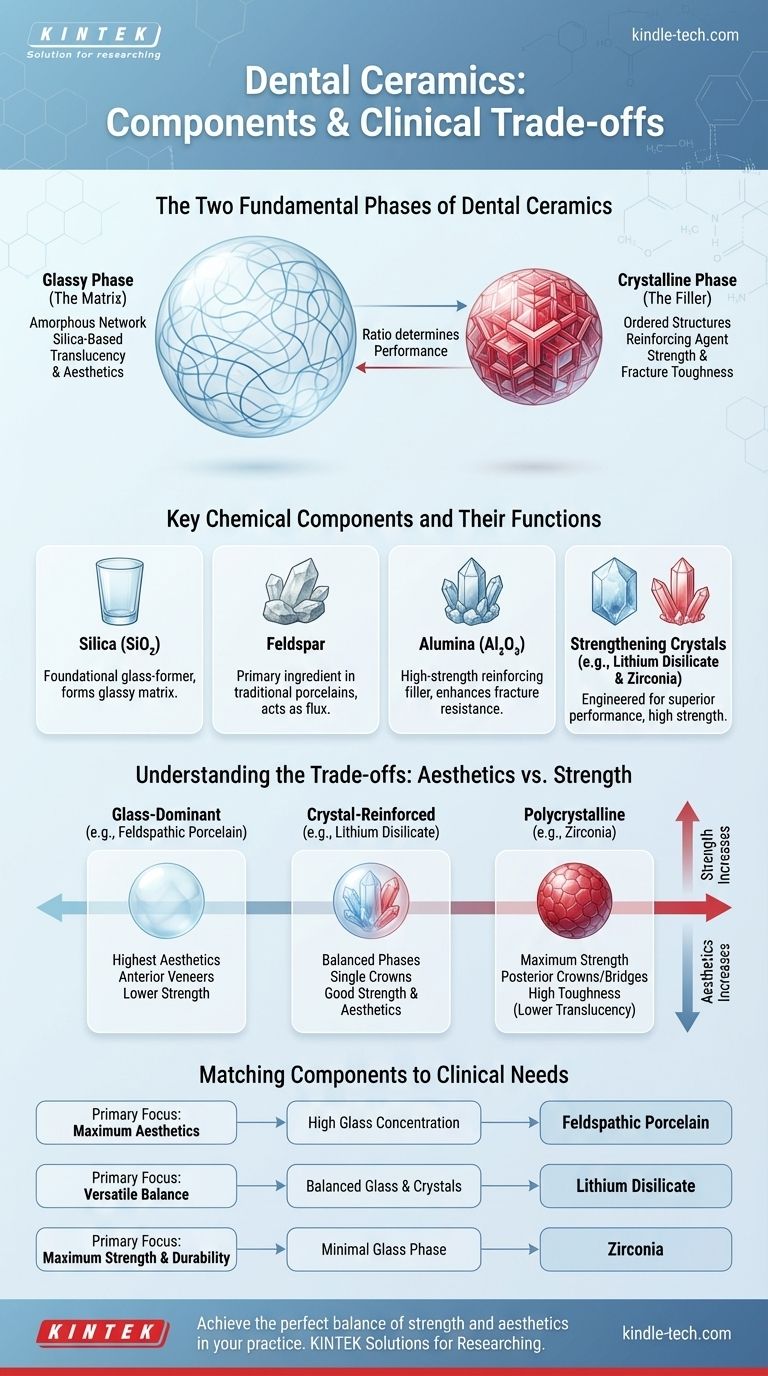
The Two Fundamental Phases of Dental Ceramics
Every dental ceramic, from traditional porcelain to modern zirconia, can be understood by examining the interplay between its two structural phases.
The Glassy Phase (The Matrix)
The glassy phase is an amorphous (non-crystalline) network of atoms, primarily based on silica. It forms the matrix that holds the entire structure together.
This glass matrix is responsible for the translucency and aesthetic properties of the ceramic. Light passes through this disordered structure more easily than through a dense crystal, mimicking the appearance of natural tooth enamel.
The Crystalline Phase (The Filler)
Embedded within the glass matrix are ordered, crystalline structures. These crystals act as the primary reinforcing agent.
The function of the crystalline phase is to increase strength and fracture toughness. When a crack starts to form in the weaker glass matrix, its path is blocked or deflected by these hard crystals, preventing catastrophic failure. They act like rebar in concrete.
Key Chemical Components and Their Functions
The specific properties of a ceramic are determined by the chemical building blocks used to create its glassy and crystalline phases.
Silica (Silicon Dioxide - SiO₂)
Silica is the foundational glass-former in most dental ceramics. Its molecules form the three-dimensional network that creates the glassy phase.
Feldspar
Feldspar is a naturally occurring mineral that has historically been the primary ingredient in dental porcelains. It is a source of both silica and alumina and acts as a flux, melting at a lower temperature to form the glass matrix. Leucite crystals often form within feldspathic porcelain upon cooling, providing reinforcement.
Alumina (Aluminum Oxide - Al₂O₃)
Alumina is a high-strength oxide used as a powerful reinforcing filler. Adding alumina crystals to the glass matrix significantly enhances the material's flexural strength and fracture resistance. In some systems, it can even be used to form a dense, opaque core over which more aesthetic porcelain is layered.
Strengthening Crystals (Lithium Disilicate & Zirconia)
Modern ceramics rely on engineered crystals for superior performance. Lithium disilicate (Li₂Si₂O₅) and Zirconium dioxide (ZrO₂), or zirconia, are the two most prominent examples.
These are not just simple fillers; they form a substantial portion of the ceramic's structure, providing exceptionally high strength that far exceeds traditional materials.
Metallic Oxides (The Modifiers and Colorants)
Small amounts of various metallic oxides are added for two critical reasons.
First, oxides like potassium oxide and sodium oxide act as fluxes or glass modifiers, lowering the melting point and making the material easier to process.
Second, coloring oxides like iron oxide, titanium oxide, and cerium oxide are added in trace amounts to provide color, shade, and opacity. This allows technicians to precisely match the restoration to the patient's natural teeth.
Understanding the Trade-offs: Aesthetics vs. Strength
The classification of dental ceramics is based on the glass-to-crystal ratio, which represents a fundamental clinical trade-off.
Glass-Dominant Ceramics (e.g., Feldspathic Porcelain)
These materials have a very high glass content and a relatively low crystalline content.
This composition results in the highest level of aesthetics and translucency, making them the ideal choice for anterior veneers where appearance is paramount. Their lower strength makes them unsuitable for high-stress applications.
Crystal-Reinforced Ceramics (e.g., Lithium Disilicate)
These materials achieve a balance between the two phases, containing a significant volume of strengthening crystals (like lithium disilicate) within a glass matrix.
This balanced composition provides both excellent strength and very good aesthetics. This versatility makes them a go-to material for a wide range of applications, including single crowns in both anterior and posterior regions.
Polycrystalline Ceramics (e.g., Zirconia)
Polycrystalline ceramics are composed almost entirely of crystals with little to no intervening glass phase.
This structure provides the maximum possible strength and fracture toughness, making zirconia the material of choice for posterior crowns and multi-unit bridges that must withstand immense chewing forces. Historically, this strength came with high opacity, but modern formulations have greatly improved their translucency.
Matching Components to Clinical Needs
Understanding these components empowers you to select the right material for the right clinical situation based on predictable properties.
- If your primary focus is maximum aesthetics: Choose a ceramic with a high concentration of the glassy phase, like feldspathic porcelain.
- If your primary focus is a versatile balance of strength and appearance: Choose a glass-ceramic with a high concentration of reinforcing crystals, like lithium disilicate.
- If your primary focus is maximum strength and durability: Choose a polycrystalline ceramic with a minimal glass phase, like zirconia.
By understanding the building blocks of dental ceramics, you can predictably translate material science into successful and long-lasting clinical outcomes.
Summary Table:
| Component | Primary Function | Key Property |
|---|---|---|
| Silica (SiO₂) | Forms the glassy matrix | Translucency, Aesthetics |
| Feldspar | Natural mineral, acts as a flux | Base for traditional porcelain |
| Alumina (Al₂O₃) | Reinforcing filler | Increases strength & fracture resistance |
| Lithium Disilicate / Zirconia | Engineered strengthening crystals | High strength for crowns & bridges |
| Metallic Oxides | Modifiers and colorants | Controls shade, opacity, and melting point |
Achieve the perfect balance of strength and aesthetics in your practice.
The right dental ceramic is crucial for a successful, long-lasting restoration. KINTEK specializes in providing high-quality lab equipment and consumables tailored to the precise needs of dental laboratories. Whether you are working with feldspathic porcelain for ultimate aesthetics or high-strength zirconia for durable bridges, having reliable materials and equipment is the foundation of excellence.
Let us help you equip your lab for success. Contact our experts today to discuss how our solutions can enhance your workflow and product quality.
Visual Guide
Related Products
- Dental Porcelain Zirconia Sintering Ceramic Furnace Chairside with Transformer
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1800℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the strongest dental ceramic? Zirconia Leads in Strength, But Is It Right for Your Case?
- What is the structure and properties of dental ceramics? Mastering the Science Behind Durable, Aesthetic Restorations
- What is the disadvantage of dental ceramic? Balancing Aesthetics with Durability and Risk
- What are the white spots on zirconia after sintering? A Guide to Diagnosing and Preventing Defects
- What is the temperature of zirconia? Mastering the Sintering Cycle for Maximum Strength
- What type of zirconia is most commonly used in dentistry? Choose Between Strength and Aesthetics
- What is furnace calibration? Ensure Precise Temperatures for Perfect Dental Restorations
- How do dental ceramic ovens process materials? Master Heat and Pressure for Perfect Restorations



















