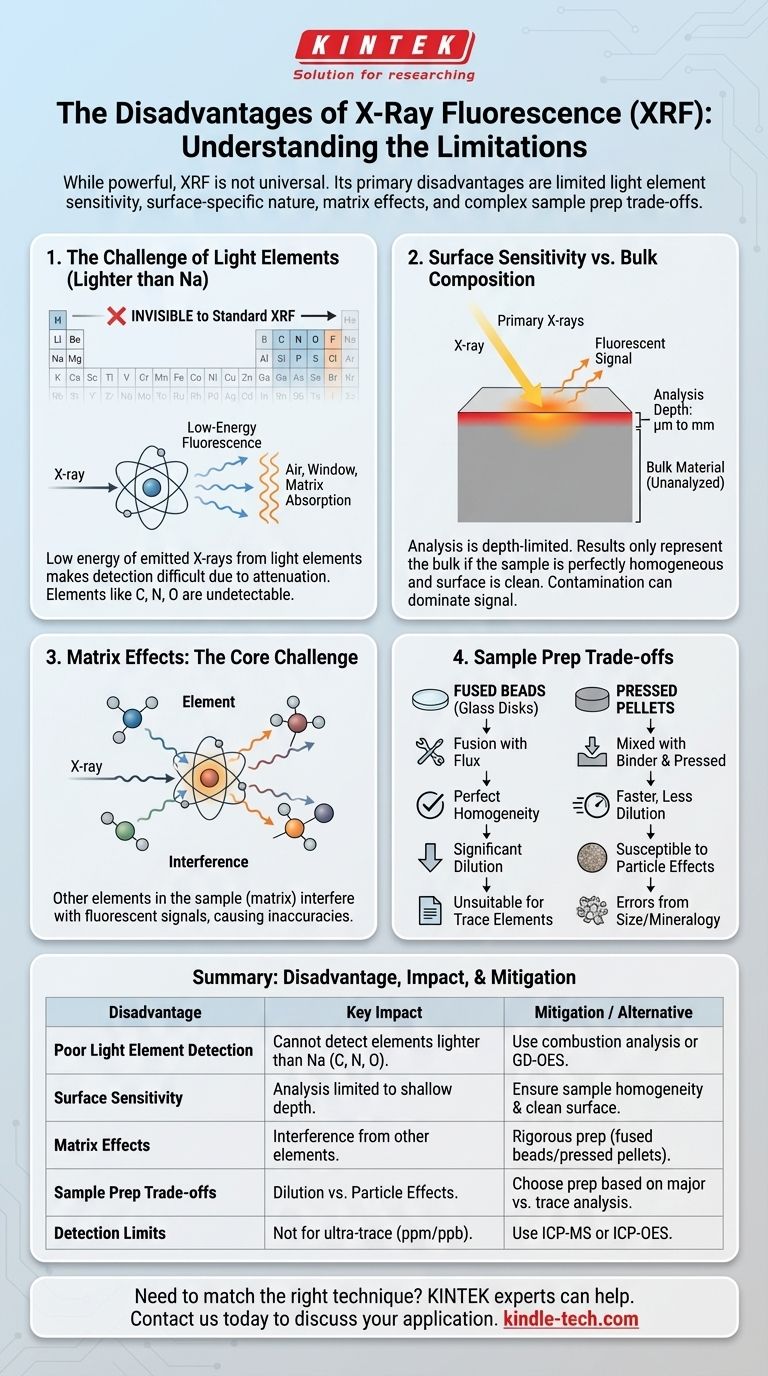
While incredibly powerful, X-Ray Fluorescence (XRF) is not a universal solution for all elemental analysis needs. Its primary disadvantages are its limited sensitivity to very light elements (those lighter than sodium), its character as a surface-sensitive technique, and the potential for complex matrix effects to interfere with quantitative accuracy. Furthermore, certain sample preparation methods, like fused beads, improve homogeneity at the cost of diluting the sample, which makes it difficult to detect trace elements.
The limitations of XRF are not flaws in the technology, but inherent physical trade-offs. Understanding these limitations—particularly regarding light elements, surface vs. bulk analysis, and the impact of sample preparation—is the key to deciding if it is the correct tool for your specific analytical challenge.

The Challenge of Light Elements
One of the most fundamental limitations of XRF is its difficulty in detecting and quantifying light elements.
The Physics of Low-Energy Fluorescence
When an X-ray strikes an atom, the atom emits its own characteristic "fluorescent" X-rays. For light elements (like Magnesium, Aluminum, and Silicon), these emitted X-rays have very low energy.
These low-energy X-rays are easily absorbed by the air between the sample and the detector, the detector window itself, or even the sample matrix. This absorption, known as attenuation, severely weakens the signal, making detection difficult and quantification unreliable.
The Analytical Blind Spot
As a direct consequence, standard XRF instruments cannot detect elements lighter than sodium (Na) on the periodic table. This includes critically important elements in many fields, such as Carbon, Nitrogen, Oxygen, and Lithium.
Surface Sensitivity vs. Bulk Composition
XRF is fundamentally a surface or near-surface analysis technique. This can be a significant disadvantage if your goal is to understand the composition of the entire, bulk material.
Limited Penetration Depth
The primary X-rays from the instrument only penetrate a very shallow depth into the sample before being absorbed or scattered. The resulting fluorescent signal only escapes from this top layer.
The effective analysis depth can range from a few micrometers to several millimeters, depending heavily on the sample's density and the energy of the X-rays. For dense materials like metal alloys, you are often analyzing less than the top 50 micrometers.
The Risk of Inaccurate Representation
This surface sensitivity means the analysis is only representative of the bulk material if the sample is perfectly homogeneous. Any surface contamination, oxidation, corrosion, or plating will dominate the signal and produce a result that does not reflect the true bulk composition.
Understanding the Trade-offs of Sample Preparation
While "non-destructive" analysis is a key advantage of XRF, achieving high accuracy often requires destructive sample preparation, which introduces its own set of trade-offs.
Matrix Effects: The Core Challenge
The "matrix" is everything in the sample that is not the element you are trying to measure. These other elements can absorb the fluorescent X-rays from your target element or emit their own X-rays that excite your target element, artificially enhancing its signal. These are known as matrix effects.
Fused Beads: Homogeneity at the Cost of Sensitivity
To eliminate matrix effects, a common technique is to fuse the sample with a flux (like lithium borate) to create a perfectly homogeneous glass disk. This solves the problem of particle size and mineralogical variation.
However, as the reference material correctly notes, this process involves significant dilution of the original sample. This dilution can push the concentration of trace elements below the instrument's detection limit, making the fused bead method unsuitable for trace element analysis.
Pressed Pellets: Speed vs. Particle Effects
Alternatively, a powdered sample can be mixed with a binder and pressed into a pellet. This is faster and involves less dilution. However, it is highly susceptible to errors from variations in particle size and mineralogy, which can scatter X-rays unpredictably.
Is XRF the Right Choice for Your Goal?
Choosing the correct analytical technique requires matching your goal to the technology's strengths and weaknesses.
- If your primary focus is rapid, non-destructive screening or material identification: XRF is an exceptional choice, but always verify your sample surface is clean and representative of the bulk material.
- If your primary focus is precise, quantitative analysis of major and minor elements: XRF is a leading technique, but you must invest in rigorous sample preparation (like fusion or pressing) and calibration with matrix-matched standards.
- If your primary focus is detecting ultra-trace elements (in the parts-per-million or billion range): XRF is generally the wrong tool due to its detection limits. You should consider Inductively Coupled Plasma (ICP-MS or ICP-OES) instead.
- If your primary focus is analyzing very light elements (like Carbon, Oxygen, or Boron): XRF is not capable of this analysis. You will need a different technique, such as combustion analysis or Glow Discharge Optical Emission Spectrometry (GD-OES).
Ultimately, mastering XRF is about knowing when to use it and when to rely on a different tool for the job.
Summary Table:
| Disadvantage | Key Impact | Mitigation / Alternative |
|---|---|---|
| Poor Light Element Detection | Cannot detect elements lighter than sodium (e.g., C, N, O). | Use combustion analysis or GD-OES. |
| Surface Sensitivity | Analysis is limited to a shallow depth (micrometers). | Ensure sample is homogeneous and surface is clean. |
| Matrix Effects | Other elements in the sample can interfere with results. | Use rigorous sample prep (fused beads/pressed pellets). |
| Sample Prep Trade-offs | Fused beads dilute sample, pressed pellets have particle effects. | Choose prep method based on analysis goals (major vs. trace elements). |
| Detection Limits | Not suitable for ultra-trace element analysis (ppm/ppb). | Use ICP-MS or ICP-OES for trace analysis. |
Need to match the right analytical technique to your specific lab challenge?
Understanding the strengths and limitations of each tool is key to achieving accurate and reliable results. The experts at KINTEK are here to help you navigate these decisions. We specialize in providing the right lab equipment and consumables for your elemental analysis needs, whether it's XRF, ICP, or other technologies.
Contact us today via our [#ContactForm] to discuss your application and discover how our solutions can enhance your lab's efficiency and accuracy.
Visual Guide
Related Products
- Laboratory Test Sieves and Sieving Machines
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Custom PTFE Teflon Parts Manufacturer for PTFE Tweezers
People Also Ask
- Why is an ultrasonic homogenizer used for LNMO samples? Ensure Accurate Particle Size Distribution Analysis
- Is lab-grown diamond business profitable? Navigate Falling Prices & Build a Profitable Brand
- What are the requirements for polymer foam templates for reticulated MAX phase ceramics? Ensure Structural Integrity
- What are the problems with magnetron sputtering? Key Challenges and How to Overcome Them
- What is the temperature of iron sintering? Achieve Optimal Sinter Quality for Your Blast Furnace
- How does a laboratory stirrer influence MOF product quality? Master Precision in Non-Solvothermal Synthesis
- How often should you change the oil in a rotary vane vacuum pump? Optimize Your Pump's Performance & Lifespan
- What are the hazards of copper brazing? Avoid catastrophic leaks and material failure



















