At first glance, the term "inert gas" suggests a substance with no effects at all. While their defining characteristic is a profound lack of chemical reactivity under normal conditions, their physical and physiological effects are significant, ranging from life-sustaining in some contexts to life-threatening in others. The primary effect of an inert gas is the physical displacement of other gases, most critically oxygen.
The term "inert" refers only to chemical non-reactivity. The true impact of these gases comes from their physical properties—such as density and solubility—which can cause dangerous physiological effects like asphyxiation and narcosis, especially in confined spaces or under high pressure.

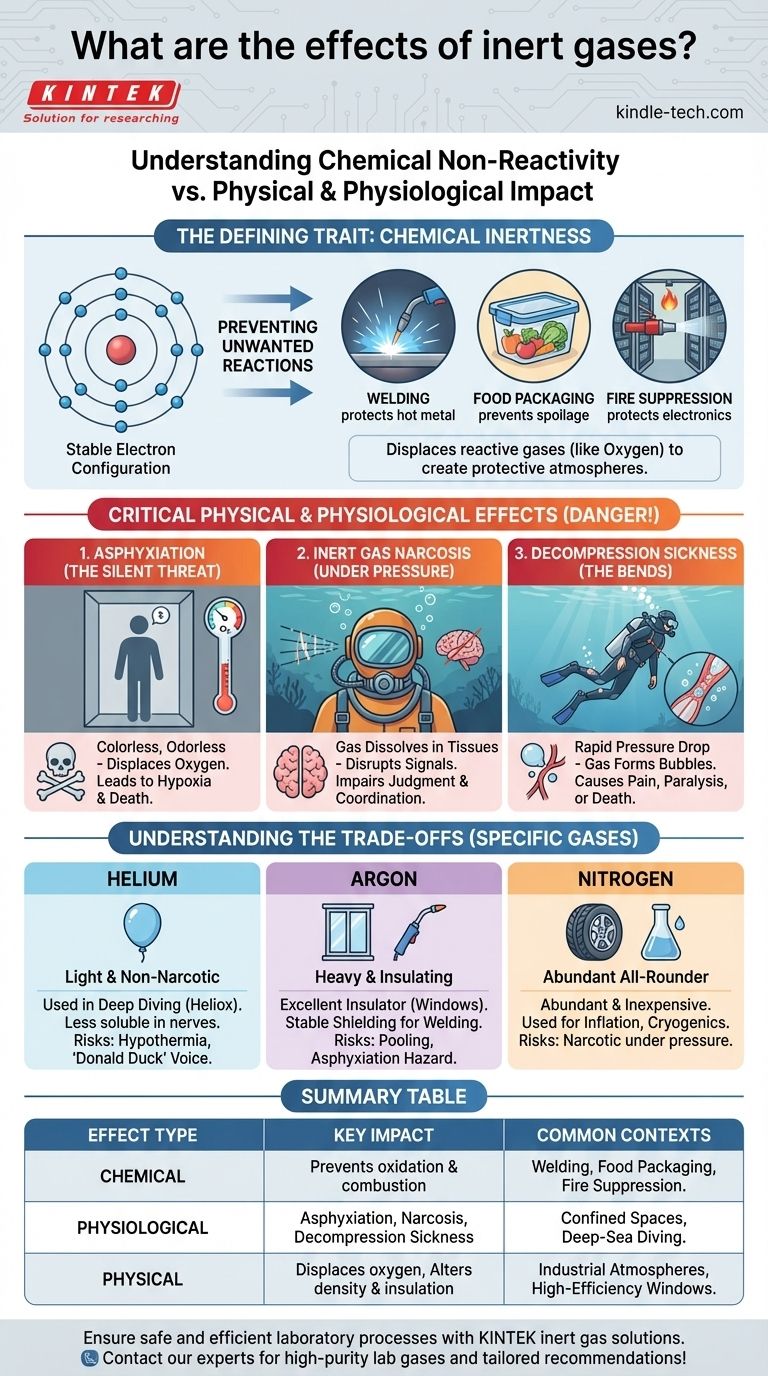
The Defining Trait: Chemical Inertness
What Makes a Gas "Inert"?
Inert gases, most notably the noble gases like helium, neon, argon, and nitrogen (which is often treated as inert), have a stable electron configuration. Their outermost electron shells are full, meaning they have very little tendency to share, gain, or lose electrons to form chemical bonds with other elements.
This chemical stability is why they are often called "non-reactive."
The Primary Consequence: Preventing Unwanted Reactions
The most common application of inert gases leverages this non-reactivity to create a protective atmosphere. By flooding an area with an inert gas, you displace reactive gases like oxygen and water vapor.
This prevents undesirable chemical processes such as oxidation (rusting) and combustion. This principle is used in welding (to protect the hot metal), food packaging (to prevent spoilage), and fire suppression systems for sensitive electronics.
Critical Physical & Physiological Effects
While chemically stable, inert gases have physical properties that create profound and often dangerous effects on biological systems and environments.
The Silent Threat: Asphyxiation by Displacement
This is the single most important effect to understand. Inert gases are colorless and odorless, providing no sensory warning of their presence.
When released into a confined or poorly ventilated space, they displace the oxygen in the air. Breathing an atmosphere with insufficient oxygen (hypoxia) leads to rapid loss of consciousness and death by asphyxiation. The human body does not have a primary reflex to detect a lack of oxygen, only a buildup of carbon dioxide, which does not occur in this scenario.
Inert Gas Narcosis
Under increased partial pressure, such as during deep-sea diving, inert gases dissolve into the body's tissues, particularly fatty tissues like those in the brain and nervous system.
This saturation of nerve membranes disrupts signal transmission, causing an intoxicating effect similar to alcohol or nitrous oxide. This phenomenon, known as inert gas narcosis, impairs judgment, reasoning, and motor coordination, posing a severe risk to divers.
Nitrogen is the classic example, but heavier gases like argon and krypton have a much stronger narcotic effect at shallower depths due to their higher solubility.
Decompression Sickness ("The Bends")
When a person returns from a high-pressure environment to a lower-pressure one too quickly, the inert gases dissolved in their tissues come out of solution and form bubbles.
These bubbles can form in joints, muscles, or blood vessels, causing extreme pain, neurological damage, paralysis, or even death. This is decompression sickness, a direct physical consequence of inert gas solubility.
Understanding the Trade-offs and Applications
The specific physical properties of each inert gas dictate its use and its associated risks. The choice is never arbitrary.
Helium: Light and Non-Narcotic
Helium is much less soluble in nerve tissue than nitrogen. For this reason, it is mixed with oxygen (as Heliox) for very deep diving to avoid the debilitating effects of narcosis.
However, helium's low density and high thermal conductivity cause divers to lose body heat much faster, increasing the risk of hypothermia. It also produces a "Donald Duck" voice effect, which can complicate communications.
Argon: Heavy and Insulating
Argon is denser than air, making it an excellent insulator. This property is used to fill the gap between panes in high-efficiency double-glazed windows.
In welding, its density provides a stable, heavy blanket of shielding gas over the weld pool, offering better protection than helium in many situations. However, this same density means it will pool in low-lying areas, creating a potent and invisible asphyxiation hazard.
Nitrogen: The Abundant All-Rounder
Nitrogen is the workhorse of inert gases because it is abundant (78% of air) and inexpensive to produce. It is used for everything from inflating tires and packaging food to creating the cryogenic temperatures of liquid nitrogen.
Its primary limitations are its narcotic potential under pressure and the fact that at very high temperatures, it is not truly inert and can form nitrides with some metals.
Making the Right Choice for Your Goal
To safely and effectively use an inert gas, you must look beyond its chemical nature and consider its physical and physiological impact.
- If your primary focus is industrial safety or fire suppression: Your main concern is oxygen displacement and the risk of asphyxiation in confined spaces.
- If your primary focus is scientific research or manufacturing: Your goal is to leverage chemical non-reactivity to create a pure, protective atmosphere for sensitive processes.
- If your primary focus is a high-pressure environment like diving: You must account for the specific narcotic potential and decompression risks associated with each gas.
Ultimately, understanding that "inert" describes chemistry, not physics or biology, is the key to mastering these uniquely useful and hazardous substances.
Summary Table:
| Effect Type | Key Impact | Common Contexts |
|---|---|---|
| Chemical | Prevents oxidation & combustion | Welding, Food Packaging, Fire Suppression |
| Physiological | Asphyxiation, Narcosis, Decompression Sickness | Confined Spaces, Deep-Sea Diving |
| Physical | Displaces oxygen, Alters density & insulation | Industrial Atmospheres, High-Efficiency Windows |
Ensure your laboratory processes are safe and efficient with the right inert gas solutions. KINTEK specializes in providing high-purity lab gases and equipment to create controlled atmospheres for welding, research, and material processing. Our experts can help you select the optimal gas—whether it's argon, helium, or nitrogen—to protect your samples, prevent contamination, and enhance safety. Contact our team today to discuss your specific application and receive a tailored recommendation!
Visual Guide
Related Products
- High-Purity Titanium Foil and Sheet for Industrial Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Cylindrical Press Mold with Scale for Lab
- Laboratory High Pressure Horizontal Autoclave Steam Sterilizer for Lab Use
- Platinum Auxiliary Electrode for Laboratory Use
People Also Ask
- What is the significance of controlled atmosphere in heat treatment? Prevent Oxidation & Guarantee Part Integrity
- Why are high-vacuum or controlled-atmosphere electric furnaces required for oxidation experiments on aerospace materials?
- What does hydrogen annealed mean? Unlock Superior Purity and Magnetic Performance
- What are hydrogen furnaces used for? Achieve Purity and Speed in High-Temperature Processing
- Why is a furnace equipped with a controlled atmosphere necessary for the preparation of active metal catalysts?
- What is an inert oven? A Guide to Oxidation-Free Thermal Processing
- What is the purpose of inert atmosphere? A Guide to Protecting Your Materials and Processes
- What is disassociated ammonia? A Cost-Effective Hydrogen Source for Heat Treating













