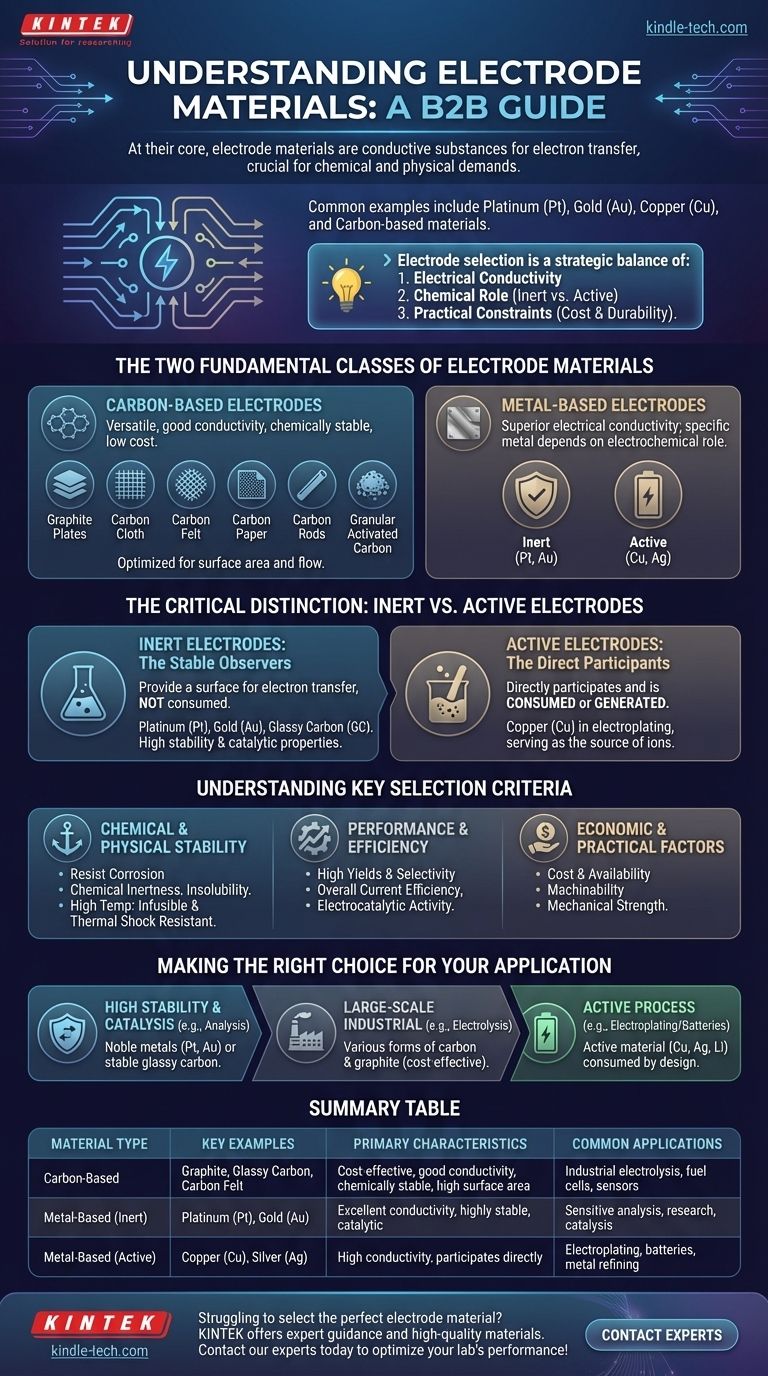
At their core, electrode materials are conductive substances, most commonly metals or various forms of carbon. The specific material is chosen based on its ability to effectively transfer electrons while meeting the unique chemical and physical demands of a given application, with common examples including platinum, gold, copper, and numerous carbon-based materials like graphite and glassy carbon.
The selection of an electrode material is not a simple choice but a strategic decision. The ideal material must balance three key factors: its electrical conductivity, its chemical role in the reaction (whether it remains inert or actively participates), and practical constraints like cost and durability.

The Two Fundamental Classes of Electrode Materials
Electrodes are broadly categorized based on their base material. The vast majority of applications use either a carbon-based or a metal-based material, each offering a distinct set of properties.
Carbon-Based Electrodes
Carbon is a versatile and widely used electrode material due to its excellent conductivity, general chemical stability, and relatively low cost.
It is available in a vast array of forms to suit different needs, such as graphite plates, carbon cloth, carbon felt, carbon paper, carbon rods, and granular activated carbon. This variety allows engineers to optimize for factors like surface area and flow.
Metal-Based Electrodes
Metals are chosen for their superior electrical conductivity. The specific metal used, however, depends entirely on the role it needs to play in the electrochemical reaction.
Common examples include highly stable metals like platinum (Pt) and gold (Au), as well as more reactive metals like copper (Cu) and silver (Ag).
The Critical Distinction: Inert vs. Active Electrodes
Beyond the base material, the most important functional difference between electrodes is whether they participate in the chemical reaction.
Inert Electrodes: The Stable Observers
An inert electrode's sole purpose is to provide a surface for the transfer of electrons without being consumed in the reaction.
Materials like platinum, gold, and glassy carbon (GC) are excellent inert electrodes. They are chosen for their chemical stability and, in many cases, their ability to catalyze a reaction without changing themselves.
Active Electrodes: The Direct Participants
An active electrode is a direct participant in the electrochemical reaction. It is made from one of the reactants and is consumed or generated during the process.
For example, in copper electroplating, a copper electrode serves as the source of copper ions that are deposited onto another surface.
Understanding the Key Selection Criteria
Choosing the right electrode involves a careful evaluation of its properties against the demands of the process.
Chemical and Physical Stability
A good electrode must withstand its operating environment. This includes resistance to corrosion, chemical inertness (if it's an inert electrode), and insolubility so it doesn't dissolve.
For high-temperature applications, such as in arc furnaces, materials must also be infusible (not melt) and resistant to thermal shock.
Performance and Efficiency
The material directly impacts the outcome of the process. Key performance indicators include the yields and selectivity of the desired chemical reaction and the overall current efficiency.
The material's inherent electrocatalytic activity can significantly speed up or enable specific reactions.
Economic and Practical Factors
Real-world applications are governed by practical constraints. The cost and availability of a material are primary concerns, especially in large-scale industrial processes.
Furthermore, the machinability of the material—how easily it can be shaped into the desired form—and its mechanical strength are critical for manufacturing and long-term reliability.
Making the Right Choice for Your Application
The optimal electrode is always application-specific. Your primary goal will dictate the best material choice.
- If your primary focus is high stability and catalysis for sensitive analysis: Noble metals like platinum and gold or highly stable glassy carbon are the standard choices.
- If your primary focus is a large-scale industrial process where cost is a major factor: Various forms of carbon and graphite often provide the most practical and cost-effective solution.
- If your primary focus is a process where the electrode is consumed (e.g., electroplating or batteries): An active electrode made from the relevant material, like copper, silver, or lithium, is required by design.
Ultimately, selecting the right electrode is about matching the material's unique properties to the specific demands of your electrochemical system.
Summary Table:
| Material Type | Key Examples | Primary Characteristics | Common Applications |
|---|---|---|---|
| Carbon-Based | Graphite, Glassy Carbon, Carbon Felt | Cost-effective, good conductivity, chemically stable, high surface area forms | Industrial electrolysis, fuel cells, sensors |
| Metal-Based (Inert) | Platinum (Pt), Gold (Au) | Excellent conductivity, highly stable, catalytic | Sensitive analysis, research, catalysis |
| Metal-Based (Active) | Copper (Cu), Silver (Ag) | High conductivity, participates directly in the reaction | Electroplating, batteries, metal refining |
Struggling to select the perfect electrode material for your lab's specific needs? The right choice is critical for the yield, efficiency, and success of your electrochemical processes. KINTEK specializes in lab equipment and consumables, offering expert guidance and a wide range of high-quality electrode materials—from cost-effective carbon to high-purity platinum. Let our specialists help you match the ideal material to your application's demands for stability, catalysis, or cost-effectiveness. Contact our experts today to discuss your requirements and optimize your lab's performance!
Visual Guide
Related Products
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Gold Disc Electrode
- Copper Sulfate Reference Electrode for Laboratory Use
- Platinum Auxiliary Electrode for Laboratory Use
People Also Ask
- What are the performance characteristics of platinum wire/rod electrodes? Unmatched Stability for Your Lab
- What is the rotating ring disk electrode method? Unlock Real-Time Reaction Analysis
- What is the RRDE in electrochemistry? Unlock Detailed Reaction Pathways with Dual-Electrode Analysis
- What are the specifications of the Platinum-Titanium Functional Electrode? Maximize Electrochemical Performance
- How should a platinum wire/rod electrode be cleaned before use? A Guide to Reliable Electrochemical Data



















