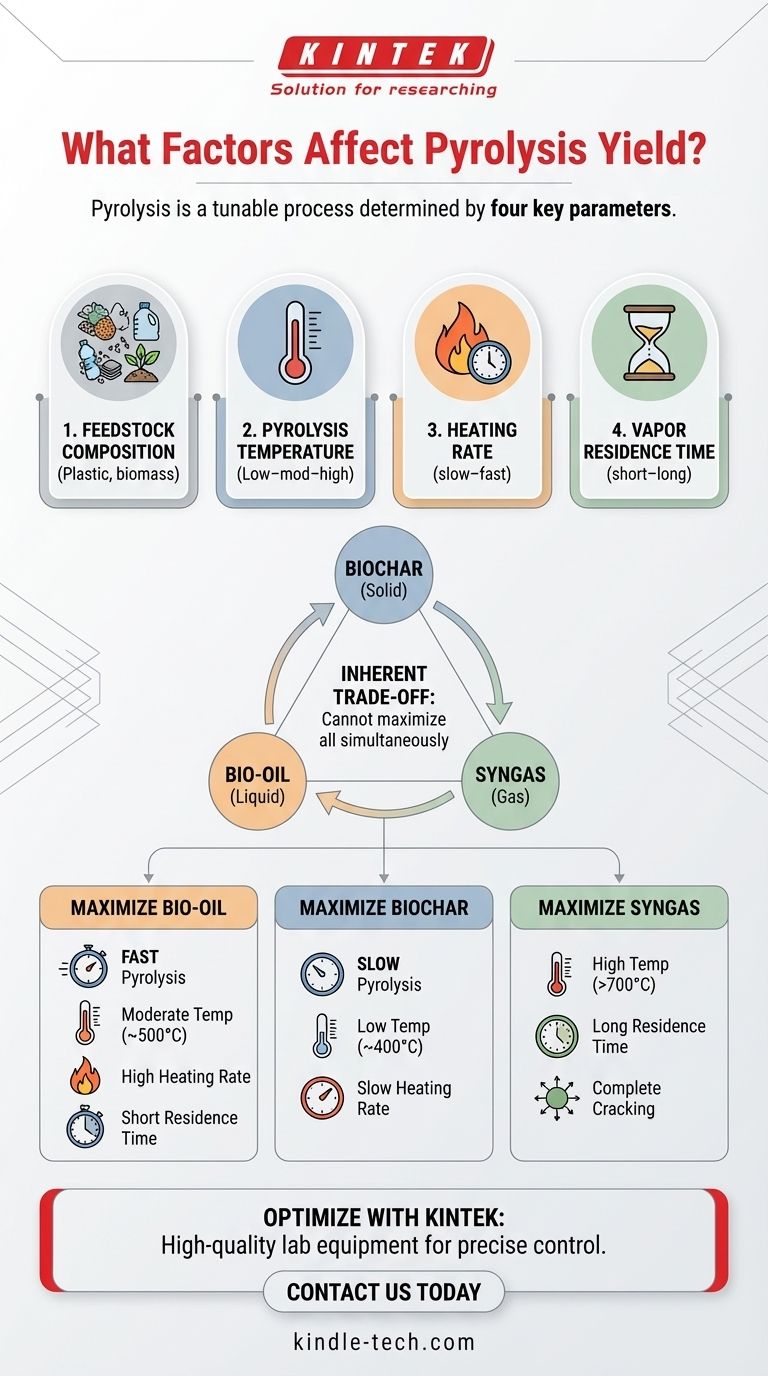
In short, pyrolysis yield is determined by four primary factors: the composition of the feedstock, the final process temperature, the rate at which the material is heated, and the amount of time the resulting vapors spend in the hot reactor zone. Understanding how to manipulate these variables is the key to controlling the output of the process, whether you are targeting solid, liquid, or gaseous products.
The core principle to understand is that pyrolysis isn't a single, fixed process. It is a highly tunable thermochemical reaction where the operational parameters act as control levers, allowing you to intentionally shift the primary output between char, oil, and gas to match your specific goal.

The Core Pillars of Pyrolysis Control
To effectively manage pyrolysis, you must understand how each operational parameter influences the chemical reactions and final product distribution. These factors work in concert, not in isolation.
### Feedstock Composition and Quality
The material you start with fundamentally defines the potential output. The chemical makeup of the feedstock serves as the raw ingredient for the final products.
For example, the type and percentage of different plastics in waste will dictate the properties of the resulting oil. Likewise, the specific composition of biomass (e.g., lignin, cellulose content) influences the characteristics of the bio-oil and biochar.
Contaminants like moisture, ash, and soil also directly impact yield, as they do not convert into valuable products and can complicate the process.
### Pyrolysis Temperature
Temperature is arguably the most dominant factor influencing the pyrolysis process. It directly controls the extent of the chemical decomposition.
At low temperatures (around 400°C), the process favors the production of solids, resulting in a higher yield of biochar.
As the temperature increases to a moderate range (around 500°C), it promotes the thermal cracking needed to produce a higher yield of liquid products (bio-oil).
At very high temperatures (above 600-700°C), secondary cracking of vapors occurs, breaking down larger molecules into smaller, non-condensable gases. This maximizes the yield of syngas.
### Heating Rate
The speed at which the feedstock is heated to the target temperature has a profound effect on the product distribution, particularly between char and liquids.
A slow heating rate (slow pyrolysis) allows more time for intermediate reactions that form solid char, thus maximizing its yield.
A high heating rate (fast pyrolysis) rapidly breaks down the material into vapors, minimizing the time available for char-forming reactions. This method is essential for maximizing the yield of liquid oil.
### Vapor Residence Time
This refers to the amount of time the hot gases and vapors remain inside the reactor before being cooled and collected.
A short vapor residence time is critical for maximizing liquid yield. Vapors are quickly removed from the hot zone and quenched, preventing them from breaking down further.
A long vapor residence time exposes the vapors to high temperatures for an extended period. This encourages secondary cracking, which breaks down the liquid components into permanent gases, thereby maximizing gas yield.
Understanding the Trade-offs
Optimizing for one product category inevitably means sacrificing the yield of another. This "product yield triangle" of char, oil, and gas is central to designing a pyrolysis operation.
### The Inherent Product Conflict
You cannot simultaneously maximize the output of all three products. The conditions that favor char (low temp, slow heating) are the opposite of those that favor liquids (moderate temp, fast heating) or gas (high temp, long residence time).
The choice of operating parameters must be a deliberate decision based on which product has the most value for your specific application.
### Process Complexity vs. Product Value
Maximizing liquid yield through fast pyrolysis requires more sophisticated and precisely controlled reactors capable of very high heating rates and rapid vapor quenching. This increases capital and operational costs.
Conversely, maximizing biochar through slow pyrolysis is often a simpler, more robust, and less energy-intensive process, but the primary output is a solid rather than a liquid fuel.
Optimizing Pyrolysis for Your Goal
Your desired end-product should dictate your entire operational strategy. Use these guidelines to align the process parameters with your objective.
- If your primary focus is maximizing bio-oil/liquid fuel: Employ fast pyrolysis with moderate temperatures (~500°C), extremely high heating rates, and a very short vapor residence time.
- If your primary focus is maximizing biochar production: Utilize slow pyrolysis with relatively low temperatures (~400°C) and a slow, gradual heating rate.
- If your primary focus is maximizing syngas generation: Use high temperatures (>700°C) and a long vapor residence time to ensure complete thermal cracking of all volatile components.
By mastering these relationships, you can transform pyrolysis from a simple decomposition process into a precise tool for chemical conversion.
Summary Table:
| Factor | Primary Impact on Yield |
|---|---|
| Feedstock Composition | Defines the potential output and quality of products (char, oil, gas). |
| Pyrolysis Temperature | Low temp favors char; moderate temp favors oil; high temp favors gas. |
| Heating Rate | Slow heating maximizes char; fast heating maximizes liquid oil. |
| Vapor Residence Time | Short time maximizes oil; long time maximizes gas via secondary cracking. |
Ready to optimize your pyrolysis process for maximum yield?
At KINTEK, we specialize in providing high-quality lab equipment and consumables for precise pyrolysis research and development. Whether you're focusing on biochar, bio-oil, or syngas production, our reactors and systems are designed to give you the control you need over temperature, heating rate, and vapor residence time.
Let our experts help you achieve your specific conversion goals. Contact us today to discuss your laboratory's pyrolysis needs!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success



















