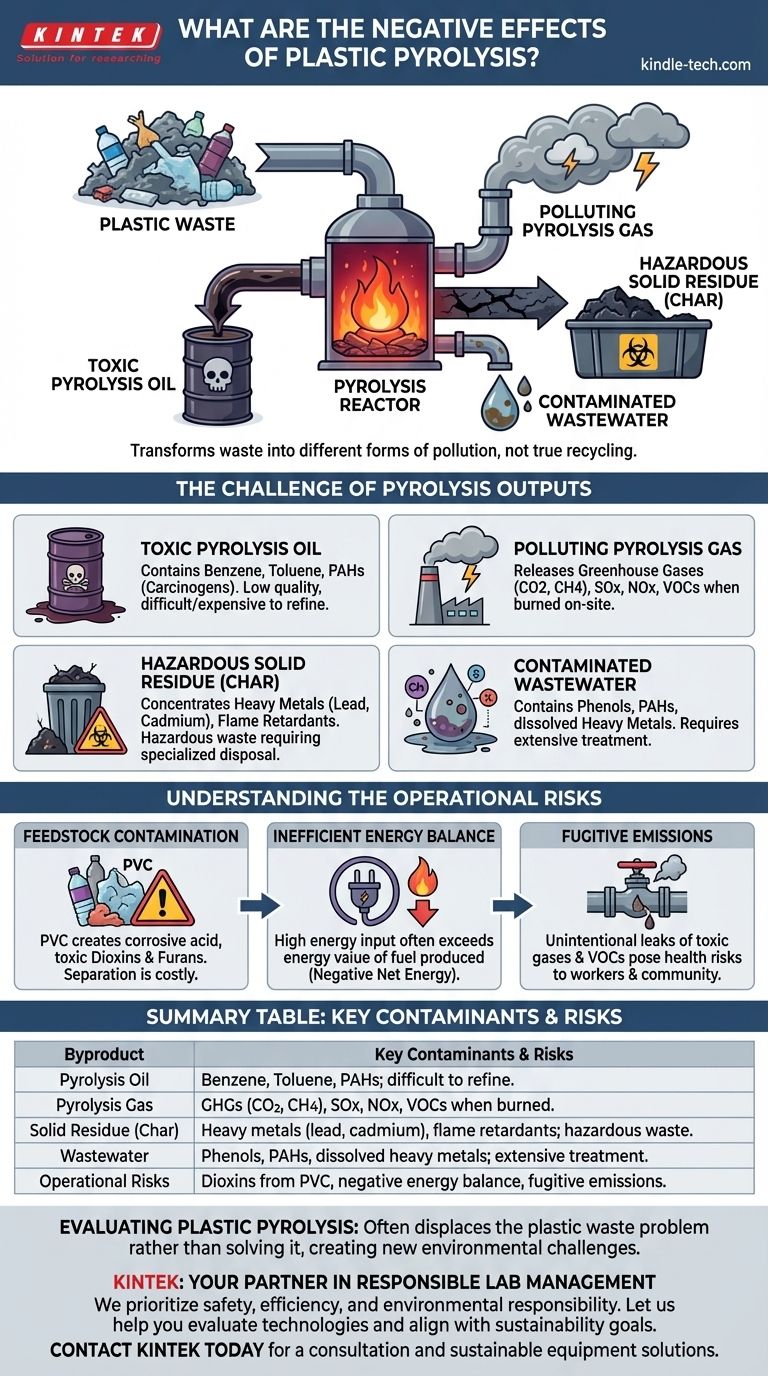
While often promoted as a chemical recycling solution, plastic pyrolysis has significant negative effects that challenge its environmental viability. The process converts plastic waste into a pyrolysis oil, a synthetic gas, and a solid residue, each of which introduces a new set of environmental and health risks. These consequences stem directly from the toxic composition of the outputs and the operational realities of the technology.
The fundamental problem with plastic pyrolysis is that it does not eliminate plastic waste but transforms it into different forms of pollution. The process generates hazardous byproducts—toxic oil, polluting gas, and contaminated solids—that can harm human health and the environment.

The Challenge of Pyrolysis Outputs
Plastic pyrolysis is a thermal decomposition process that breaks down long polymer chains into smaller, less complex molecules. However, "less complex" does not mean "harmless." The outputs are often contaminated with toxins that were either in the original plastic or are created during the reaction.
Toxic Pyrolysis Oil
The primary liquid output, often called pyrolysis oil or "pyr-oil," is frequently marketed as a substitute for crude oil. However, it is a complex, low-quality mixture that is difficult and expensive to refine.
This oil is consistently found to be contaminated with hazardous substances, including benzene, toluene, and polycyclic aromatic hydrocarbons (PAHs), which are known carcinogens. Using this oil as a fuel without significant upgrading releases these toxins and other harmful pollutants into the air.
Polluting Pyrolysis Gas
The non-condensable gas, or "syn-gas," produced during pyrolysis is typically burned on-site to provide energy for the process itself.
While this may seem efficient, the combustion of this gas can release a host of pollutants. These include greenhouse gases like carbon dioxide and methane, as well as acid gases (SOx, NOx) and volatile organic compounds (VOCs) if not captured by sophisticated and costly air pollution control systems.
Hazardous Solid Residue (Char)
The solid byproduct of pyrolysis is a carbonaceous residue known as char. This is not a benign material like charcoal.
The char acts as a sink for contaminants present in the plastic feedstock, concentrating heavy metals like lead, cadmium, and chromium, as well as flame retardants and other chemical additives. This makes the char a hazardous waste that requires specialized disposal in secure landfills, representing a significant cost and environmental liability.
Contaminated Wastewater
If the plastic feedstock contains any moisture, the process generates wastewater. This water comes into contact with the decomposing plastic and becomes contaminated with phenols, PAHs, and dissolved heavy metals. Discharging this water requires extensive on-site treatment to avoid polluting local water sources.
Understanding the Operational Risks
Beyond the direct outputs, the day-to-day operation of pyrolysis facilities presents its own set of challenges that contribute to its negative impact.
Feedstock Contamination
Real-world plastic waste is never pure. The presence of materials like PVC is a major problem, as its chlorine content can create highly corrosive hydrochloric acid and extremely toxic dioxins and furans during heating. Separating plastics to the required purity is technically difficult and economically prohibitive.
Inefficient Energy Balance
Pyrolysis is an energy-intensive process requiring high temperatures to break down plastics. The energy required to run the facility can often be greater than the energy value of the fuels it produces, resulting in a negative net energy balance. This undermines its claim as an energy production method.
Fugitive Emissions
Pyrolysis facilities are complex chemical plants with the potential for fugitive emissions. These are unintentional leaks of toxic gases and VOCs from pipes, valves, and other equipment, posing a direct health risk to facility workers and the surrounding community.
Making the Right Choice for Your Goal
Evaluating plastic pyrolysis requires looking past the marketing claims and focusing on the full life cycle of its products and byproducts.
- If your primary focus is true circularity: Recognize that pyrolysis is a destructive process that downcycles plastic into fuel to be burned, not a true recycling method that creates new plastic.
- If your primary focus is waste volume reduction: Acknowledge that while it reduces solid waste volume, it transforms it into hazardous materials that require careful and costly management.
- If your primary focus is energy generation: Scrutinize the net energy balance and account for the substantial cost of the pollution control equipment needed to safely use its low-grade fuel products.
Ultimately, a critical evaluation reveals that plastic pyrolysis often displaces the plastic waste problem rather than solving it, creating a new set of complex environmental challenges.
Summary Table:
| Byproduct | Key Contaminants & Risks |
|---|---|
| Pyrolysis Oil (Pyr-oil) | Benzene, Toluene, PAHs (carcinogens); difficult/expensive to refine. |
| Pyrolysis Gas (Syn-gas) | Greenhouse gases (CO2, CH4), SOx, NOx, VOCs when burned. |
| Solid Residue (Char) | Heavy metals (lead, cadmium), flame retardants; classified as hazardous waste. |
| Wastewater | Phenols, PAHs, dissolved heavy metals; requires extensive treatment. |
| Operational Risks | Dioxins from PVC, negative energy balance, fugitive emissions. |
Make an informed choice for your laboratory's sustainability goals.
Plastic pyrolysis presents significant environmental and health risks that can undermine your organization's commitment to safety and true circularity. Before investing in waste management solutions, it's crucial to understand the full lifecycle impact of the technologies you adopt.
KINTEK is your partner in responsible lab management. We specialize in providing laboratory equipment and consumables that prioritize safety, efficiency, and environmental responsibility. Let our experts help you evaluate technologies and implement solutions that align with your sustainability and operational safety standards.
Contact KINTEL today for a consultation and discover how we can support your laboratory's needs with reliable, safe, and sustainable equipment.
Visual Guide
Related Products
- Customizable Laboratory High Temperature High Pressure Reactors for Diverse Scientific Applications
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Wall Mounted Water Distillation Unit
- High Temperature Constant Temperature Heating Circulator Water Bath Chiller Circulator for Reaction Bath
People Also Ask
- What is a calcination furnace? A Guide to High-Temperature Chemical Transformation
- What is the heat required for pyrolysis? Mastering Energy Input for Optimal Biochar, Bio-Oil, or Syngas
- What is the future potential of pyrolysis and gasification? Unlocking Value from Waste
- How does pyrolysis help the environment? Transform Waste into Renewable Energy and Carbon Sequestration
- What can pyrolysis oil be used for? A Guide to Fuel, Chemicals, and Waste Valorization
- What is the difference between pyrolysis and gasification of biomass? Choose the Right Process for Your Goals
- What are the benefits of pyrolysis of plastic waste? Turn Waste into Fuel and Reduce Landfill Volume
- What is the use of biochar from pyrolysis? Unlock its potential as fuel, material, and soil amendment


















