The most common precursors for carbon nanotubes (CNTs) are simple hydrocarbon gases. Methane, ethylene, and especially acetylene serve as the direct source of carbon atoms that assemble into the nanotube structure during synthesis.
The choice of a carbon precursor is a critical decision that directly influences the energy requirements and overall efficiency of the entire synthesis process, with simpler molecules often requiring more energy to break down.

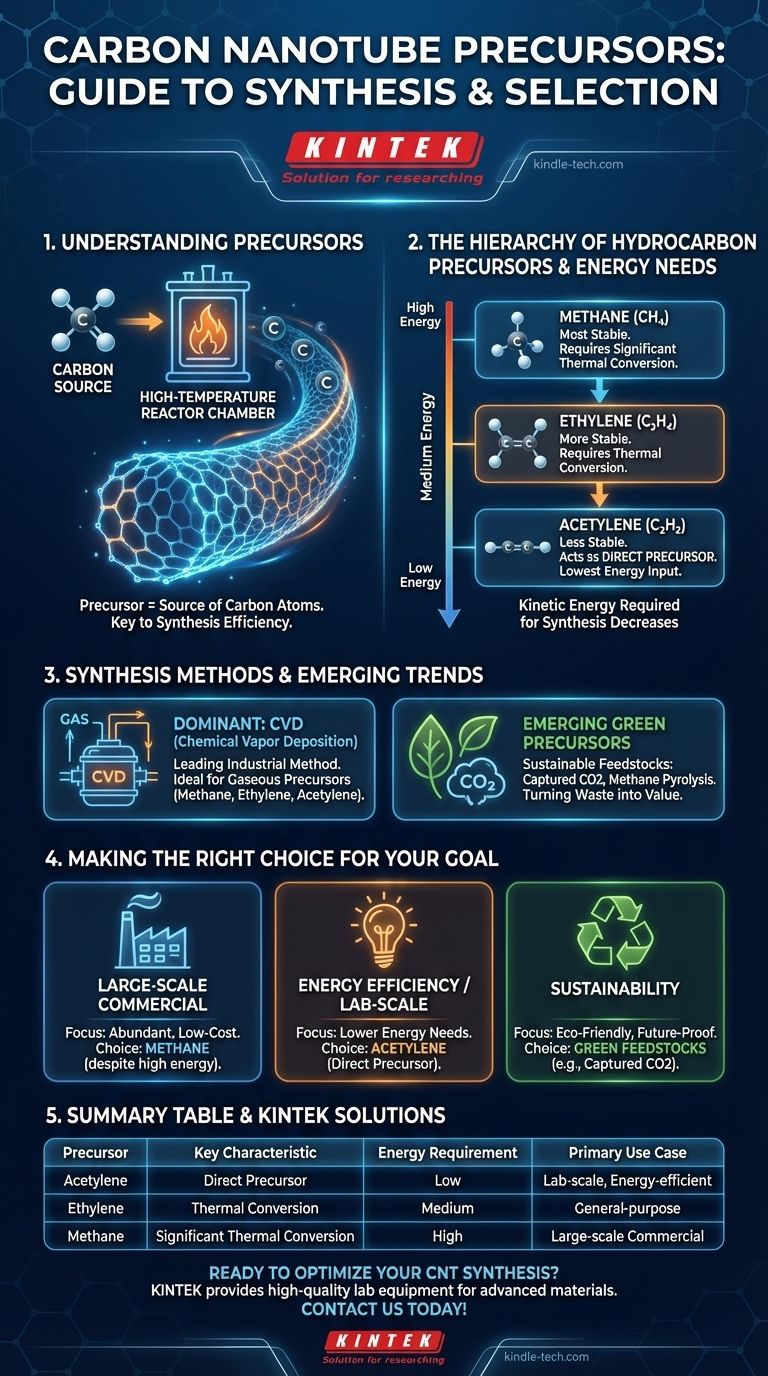
Understanding Precursors and Process Conditions
A precursor is the foundational raw material that provides the necessary elements for a chemical synthesis. For carbon nanotubes, the precursor is simply the source of carbon atoms.
The Role of a Carbon Source
The precursor gas is introduced into a high-temperature reactor, where it decomposes. This process, often aided by a metal catalyst, frees carbon atoms to self-assemble into the unique hexagonal lattice structure of a nanotube.
Key Synthesis Parameters
The success of this conversion depends on a delicate balance of operating parameters. The temperature, the concentration of the carbon source, and the residence time (how long the gas stays in the reactor) are the three most critical factors that dictate the efficiency of CNT production.
The Hierarchy of Common Hydrocarbon Precursors
Not all hydrocarbon precursors behave the same way. Their chemical stability dictates the amount of energy required to initiate the CNT growth process.
Acetylene: The Direct Precursor
Acetylene is unique because it can act as a direct precursor for carbon nanotubes. Its chemical structure is less stable, allowing it to decompose and provide carbon atoms without significant additional energy input for thermal conversion.
Methane and Ethylene: Requiring Thermal Conversion
In contrast, methane and ethylene are more stable molecules. They rely on thermal conversion processes, meaning they require a substantial amount of energy to break their chemical bonds before carbon atoms are available for CNT synthesis.
The Energy Requirement Scale
This creates a clear energy hierarchy among the common precursors. The kinetic energy needed for successful synthesis follows this order:
Methane > Ethylene > Acetylene
Methane, being the most stable, demands the most energy to break down, while acetylene requires the least.
How Synthesis Methods Influence Precursor Choice
While older methods exist, the dominant commercial process for making CNTs today heavily influences which precursors are used.
Chemical Vapor Deposition (CVD)
Chemical Vapor Deposition (CVD) is the leading industrial method for CNT production. This process is well-suited for gaseous precursors like methane, ethylene, and acetylene, making them the workhorses of the industry.
Legacy Methods
Traditional methods like laser ablation and arc discharge, which involve vaporizing a solid carbon target, are less common for large-scale production today compared to the more scalable CVD approach.
Emerging Green Precursors
Research is actively exploring more sustainable feedstocks. These emerging methods aim to create CNTs from waste or captured carbon, representing a significant shift from traditional hydrocarbon sources. This includes using carbon dioxide captured via electrolysis or leveraging methane pyrolysis.
Making the Right Choice for Your Goal
The ideal precursor is entirely dependent on the primary objective of the synthesis.
- If your primary focus is large-scale commercial production: Abundant and low-cost feedstocks like methane are often chosen, despite their higher energy requirements.
- If your primary focus is energy efficiency or lab-scale synthesis: Acetylene is a strong candidate due to its ability to act as a direct precursor with lower energy needs.
- If your primary focus is sustainability: Emerging green feedstocks like captured CO2 or pyrolyzed methane are the future, turning waste streams into high-value materials.
Ultimately, the selection of a carbon precursor fundamentally defines the cost, efficiency, and environmental impact of carbon nanotube synthesis.
Summary Table:
| Precursor | Key Characteristic | Energy Requirement | Primary Use Case |
|---|---|---|---|
| Acetylene | Acts as a direct precursor | Low | Lab-scale, energy-efficient synthesis |
| Ethylene | Requires thermal conversion | Medium | General-purpose production |
| Methane | Requires significant thermal conversion | High | Large-scale commercial production |
Ready to Optimize Your Carbon Nanotube Synthesis?
Choosing the right precursor is critical for achieving the desired quality, yield, and cost-efficiency in your CNT production. KINTEK specializes in providing high-quality lab equipment and consumables tailored for advanced materials synthesis, including carbon nanotubes.
Our experts can help you select the right tools and provide insights to scale your process effectively. Contact us today to discuss your specific needs and let KINTEK be your partner in innovation.
Get in touch with our experts now!
Visual Guide
Related Products
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- CVD Diamond Domes for Industrial and Scientific Applications
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Large Vertical Graphite Vacuum Graphitization Furnace
People Also Ask
- What is sputtering of thin films? A Guide to Precision Thin Film Deposition
- What are the deposition materials? A Guide to Metals, Ceramics, and Compounds for Thin Films
- What nanomaterials are synthesized by chemical vapor deposition? Building High-Performance Materials with Precision
- What are the applications of sputtering deposition? Achieve Superior Thin Films for Electronics, Optics & Tools
- What are the characterization of single-walled carbon nanotubes? Essential Techniques for SWCNT Analysis
- What are the environmental applications of carbon nanotubes? Boost Efficiency & Sustainability
- How does the sputtering method work? A Guide to Thin-Film Deposition via Atomic Bombardment
- What are optical coatings made of? Metals, Dielectrics & Polymers for Light Control



















