In short, X-ray Fluorescence (XRF) is an exceptionally versatile technique capable of analyzing a vast range of materials. Samples can be analyzed as solids, pressed powders, fused beads, or liquids, and include everything from crushed rocks, metal alloys, and cement to plastics, soils, biological materials, and residues from solutions.
The critical factor in X-ray fluorescence is not what you can analyze, but how you prepare it. The accuracy and reliability of your results are almost entirely dependent on the quality of your sample preparation, as this ensures the small area being analyzed is a true representation of the entire material.

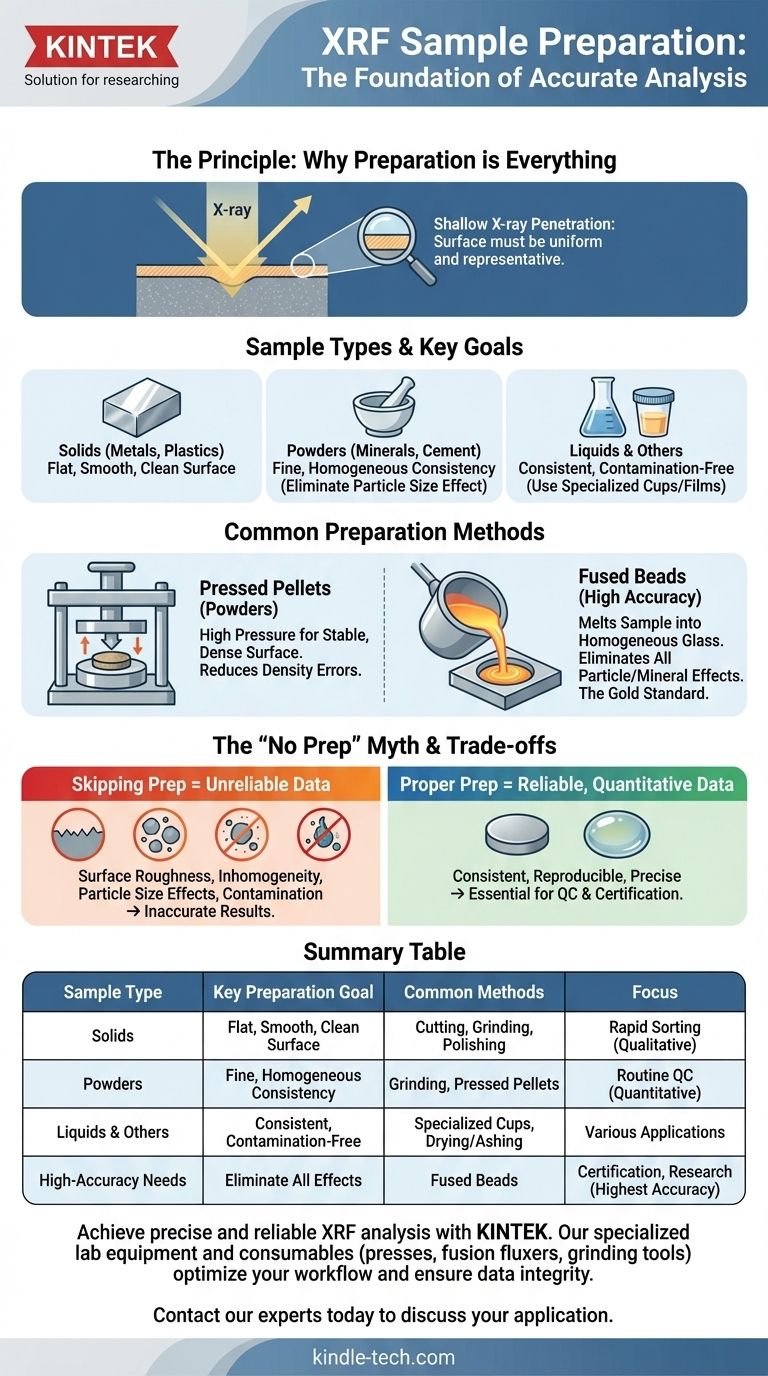
The Principle: Why Preparation is Everything
XRF works by bombarding a sample with X-rays, causing the atoms within to emit their own "characteristic" X-rays. By measuring these emitted X-rays, the instrument identifies the elements present and their concentrations.
However, the X-ray beam only penetrates a very shallow layer of the sample's surface. If that surface is not perfectly uniform and representative of the bulk material, the results will be inaccurate. This is the fundamental reason why sample preparation is the most important step in the entire analytical process.
Solids: Surface Integrity is Paramount
Solid samples, such as metal alloys, plastics, or ceramics, are often analyzed directly. For this to be successful, the surface must be ideal.
The surface needs to be flat, smooth, and clean. Any roughness, contamination, or oxidation will absorb or scatter the X-rays unpredictably, introducing significant error. Preparation often involves cutting, grinding, or polishing the sample to create a uniform analysis surface.
Powders: Homogeneity is the Goal
A huge variety of materials are analyzed as powders, including minerals, ores, pharmaceuticals, cement, and soil. The goal here is to eliminate inconsistencies caused by particle size and mineral structure.
Grinding the sample into a fine, uniform powder ensures that individual grains don't disproportionately affect the measurement. Larger, denser particles can shield lighter elements from the X-ray beam, a phenomenon known as the "particle size effect," leading to incorrect readings.
Liquids and Other Forms: Containment and Consistency
XRF can also analyze liquids, slurries, or even ashed organic matter. These samples are typically held in a specialized sample cup with a thin, X-ray transparent film at the bottom.
The key challenge is ensuring consistency and preventing contamination. For liquids, this means making sure no solids have settled out. For other materials, such as fibers or alternative fuels, preparation might involve ashing or drying to create a more stable and concentrated sample for analysis.
Common Preparation Methods
While you can simply place a loose powder in a cup, a few standard methods are used to guarantee high-quality, reproducible data.
Pressed Pellets
This is the most common method for powdered samples. The fine powder is mixed with a binder and then compressed under high pressure to form a dense, durable pellet with a perfectly flat and stable analytical surface. This method drastically reduces errors from inconsistent sample density.
Fused Beads
Considered the "gold standard" for accuracy, fusion is used when the highest precision is required. The sample is mixed with a lithium borate flux and heated in a crucible to over 1000°C, completely dissolving the sample into a molten glass.
This molten glass is then cast into a perfectly homogeneous disc. This process completely eliminates all particle size and mineralogical effects, providing the most accurate and repeatable results possible with XRF.
Understanding the Trade-offs: The Myth of "No Prep"
XRF is often promoted as a simple, non-destructive "point-and-shoot" technique. While this is true for basic material identification, it is a dangerous misconception for anyone needing reliable, quantitative data.
Why Skipping Preparation Leads to Failure
Neglecting proper preparation introduces errors that make the results unreliable. The primary sources of inaccuracy are:
- Surface Roughness: Scatters the X-ray beam.
- Inhomogeneity: The analyzed spot doesn't represent the whole sample.
- Particle Size Effects: Large or dense particles block the signals from other elements.
- Contamination: Oils, dust, or oxides on the surface are analyzed along with the sample.
Accuracy vs. Speed
Choosing a preparation method is always a trade-off between the time invested and the data quality required. A quick analysis of a loose powder might tell you if a piece of steel is a 300-series or 400-series stainless, but it won't give you the precise chromium and nickel content needed for quality control.
The time saved by skipping preparation is often lost many times over when you are forced to make critical decisions based on faulty data.
Matching Your Method to Your Analytical Goal
The right preparation technique depends entirely on your objective.
- If your primary focus is rapid sorting or basic material identification: A simple analysis of a cleaned solid surface or loose powder may be sufficient for qualitative answers.
- If your primary focus is routine process and quality control: Using pressed pellets provides the consistent, reproducible, and quantitative data needed to monitor production.
- If your primary focus is certification, research, or method development: The fused bead method is the definitive choice for achieving the highest possible accuracy and eliminating analytical ambiguity.
Ultimately, mastering sample preparation is how you unlock the true analytical power and precision of X-ray fluorescence.
Summary Table:
| Sample Type | Key Preparation Goal | Common Methods |
|---|---|---|
| Solids (Metals, Plastics) | Flat, smooth, clean surface | Cutting, Grinding, Polishing |
| Powders (Minerals, Cement) | Fine, homogeneous consistency | Grinding, Pressed Pellets |
| Liquids & Others (Slurries, Ashed Organics) | Consistent, contamination-free | Specialized Cups, Drying/Ashing |
| High-Accuracy Needs (Certification, Research) | Eliminate all mineral/particle effects | Fused Beads |
Achieve precise and reliable XRF analysis with KINTEK.
Proper sample preparation is the foundation of accurate results. Whether you're working with metals, powders, or complex materials, KINTEK's specialized lab equipment and consumables—including presses, fusion fluxers, and grinding tools—are designed to meet your specific needs.
Let us help you optimize your workflow and ensure data integrity.
Contact our experts today to discuss your application and discover the right solution for your laboratory.
Visual Guide
Related Products
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- XRF & KBR steel ring lab Powder Pellet Pressing Mold for FTIR
- Laboratory Manual Hydraulic Pellet Press for Lab Use
People Also Ask
- What role does a laboratory hydraulic press play in pellet-type electrode fabrication? Enhancing Solid-State Performance
- Why is a laboratory hydraulic press essential for Ca3Co4O9 pelletizing? Optimize Pre-Sintering Mass Transport
- How do you prepare samples for infrared spectroscopy? Master Solid, Liquid & Gas Techniques
- What is an example of a hydraulic press? Discover the Power of Laboratory Sample Preparation
- What is the use of manual hydraulic press? A Cost-Effective Tool for Lab Sample Preparation



















