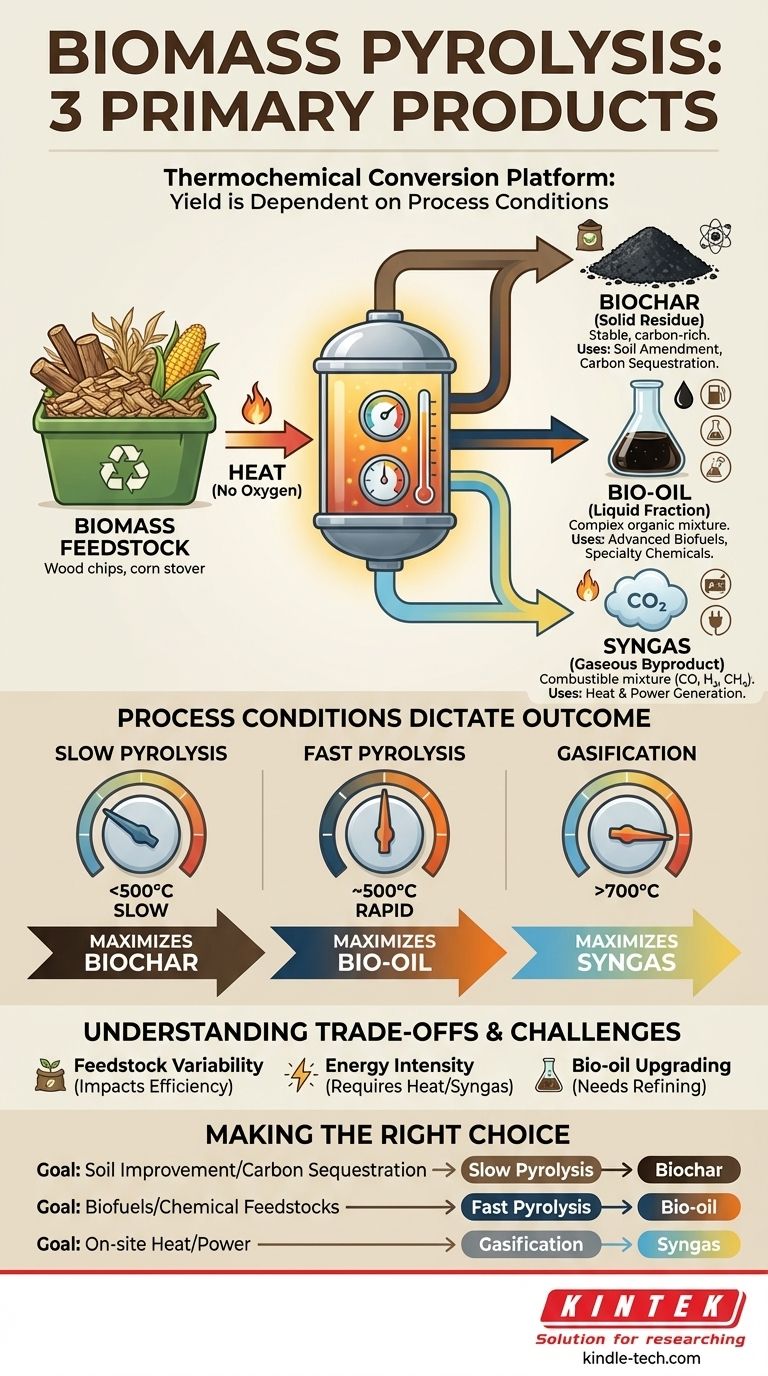
In short, the pyrolysis of biomass yields three primary products in distinct physical states: a solid residue (biochar), a liquid condensate (bio-oil), and a non-condensable gas (syngas). The specific proportions of these three products are not fixed; they are highly dependent on the type of biomass used and, most importantly, the conditions under which the pyrolysis is performed.
Biomass pyrolysis is not a single, rigid process but a flexible thermochemical conversion platform. By controlling factors like temperature and heating rate, you can intentionally shift the output to maximize the yield of either solid biochar, liquid bio-oil, or combustible syngas, tailoring the process to a specific economic or environmental goal.

Deconstructing the Three Products
Pyrolysis is the thermal decomposition of a material in the absence of oxygen. When applied to biomass, this process breaks down complex organic polymers like cellulose and lignin into simpler, more valuable components.
Biochar: The Solid Residue
Biochar is the stable, carbon-rich solid that remains after the volatile components of the biomass have been driven off. It is functionally similar to common charcoal.
Its primary value lies in its use as a soil amendment, where it can improve water retention and soil structure. It is also a method of carbon sequestration, as the carbon locked in the biochar is highly resistant to decomposition.
Bio-oil: The Liquid Fraction
As the biomass heats, volatile compounds are released as a vapor. When this vapor is cooled and condensed, it forms a dark, viscous liquid known as bio-oil (or pyrolysis oil).
This liquid is a complex mixture of water, acids, alcohols, and hundreds of other organic compounds. While it requires significant refining, bio-oil is a promising feedstock for producing advanced biofuels and specialty chemicals.
Syngas: The Gaseous Byproduct
Syngas, short for synthesis gas, is the portion of the released vapor that does not condense into a liquid.
It is a mixture of combustible gases, primarily carbon monoxide (CO), hydrogen (H₂), and methane (CH₄), along with carbon dioxide (CO₂). This gas can be combusted directly to provide the heat needed to sustain the pyrolysis reaction itself or used to generate electricity.
How Process Conditions Dictate the Outcome
You can think of the different pyrolysis methods as dials that you can turn to favor the production of one product over the others. The two most important "dials" are temperature and heating rate.
Slow Pyrolysis: Maximizing Biochar
This process uses relatively low temperatures (below 500°C) and slow heating rates. By heating the biomass slowly over a longer period, the process maximizes the yield of the solid biochar, often achieving yields of around 35%.
This is the oldest form of pyrolysis and is analogous to traditional methods of making charcoal for cooking or metallurgy.
Fast Pyrolysis: Maximizing Bio-oil
This process is engineered to produce the highest possible liquid yield. It uses moderate temperatures (around 500°C) but extremely high heating rates and very short vapor residence times (typically less than 2 seconds).
The goal is to rapidly break down the biomass into vapors and then quickly cool and condense them before they can break down further into gases. This is the key pathway for producing liquid biofuels from biomass.
Gasification: Maximizing Syngas
While sometimes considered a separate process, gasification operates on the same principles but at much higher temperatures (typically above 700°C).
At these temperatures, the liquid and solid products are "cracked" into smaller, gaseous molecules. The primary goal of gasification is to convert nearly all the biomass into a high-energy syngas.
Understanding the Trade-offs
While powerful, pyrolysis is not a silver bullet. Acknowledging its challenges is key to understanding its practical application.
Feedstock Variability
The composition and moisture content of the biomass feedstock (e.g., wood chips, corn stover, switchgrass) significantly impact the process efficiency and the exact chemical makeup of the final products.
Energy Intensity
Reaching and maintaining the high temperatures required for pyrolysis, especially the rapid heating rates for fast pyrolysis, is an energy-intensive process. A plant's overall efficiency depends on its ability to use the syngas byproduct to power its own operations.
Bio-oil Upgrading
Bio-oil is not a "drop-in" replacement for petroleum crude oil. It is highly acidic, chemically unstable, and has a high oxygen and water content. It requires significant and often costly catalytic upgrading to be refined into stable transportation fuels.
Making the Right Choice for Your Goal
The optimal pyrolysis strategy is defined entirely by your end goal.
- If your primary focus is carbon sequestration or soil improvement: Slow pyrolysis is the most direct route, as it maximizes the production of stable, carbon-rich biochar.
- If your primary focus is producing advanced biofuels or chemical feedstocks: Fast pyrolysis is the target process, designed specifically to maximize the yield of liquid bio-oil.
- If your primary focus is generating on-site heat and power from waste: Conventional pyrolysis or gasification are effective, as they produce combustible syngas that can power a generator or furnace.
Understanding these distinct pathways allows you to see pyrolysis not as a single outcome, but as a versatile tool for converting biomass into the most valuable product for your specific application.
Summary Table:
| Product | Physical State | Primary Use | Maximized By Process |
|---|---|---|---|
| Biochar | Solid | Soil amendment, carbon sequestration | Slow Pyrolysis (<500°C, slow heating) |
| Bio-oil | Liquid | Biofuel, chemical feedstock | Fast Pyrolysis (~500°C, rapid heating) |
| Syngas | Gas | On-site heat and power generation | Gasification (>700°C) |
Ready to convert your biomass into valuable products? The right pyrolysis equipment is critical for optimizing your yield of biochar, bio-oil, or syngas. KINTEK specializes in high-quality lab equipment and consumables for developing and scaling pyrolysis processes. Our experts can help you select the right tools for your research and production goals. Contact our team today to discuss your laboratory's specific needs!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What are the different types of pyrolysis technology? Choose the Right Process for Your Output Goal
- Is pyrolysis a green process? Unlocking Sustainable Waste-to-Energy Solutions
- What is the difference between refining and calcination? A Guide to Material Processing Stages
- What is biomass pyrolysis used for? Turn Waste into Renewable Energy and Valuable Products
- What are the applications of calcination? A Guide to Thermal Processing in Industry
- What are the advantages of using a rotary reactor for ALD on copper powders? Superior Coating for Cohesive Materials
- What is the efficiency of pyrolysis? Unlocking the True Performance of Your Pyrolysis Process
- What is the structure of a rotary hearth furnace? A Guide to Continuous, Uniform Heating



















