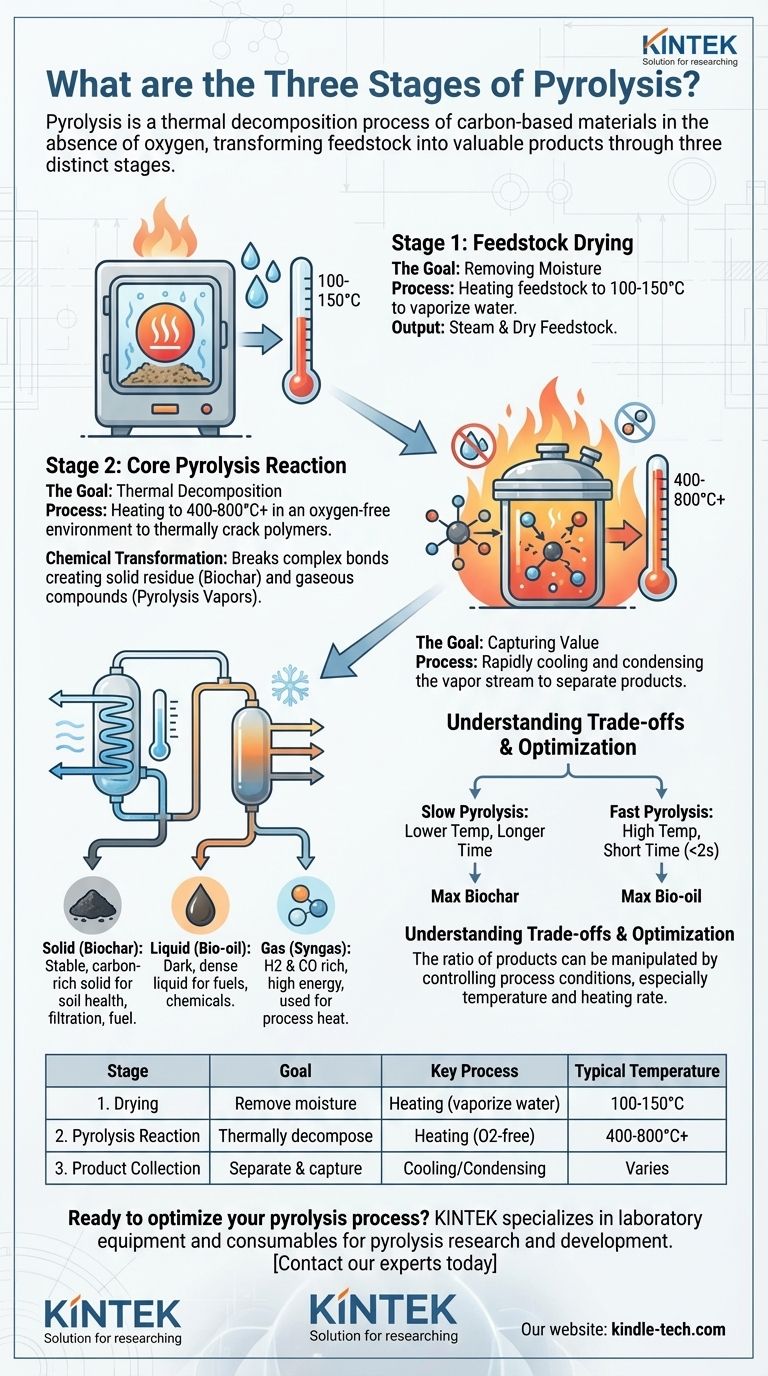
At its core, pyrolysis is a thermal decomposition process that breaks down carbon-based materials at high temperatures in the absence of oxygen. It consists of three distinct stages: drying to remove moisture, the main pyrolysis reaction to decompose the material, and finally, the condensation and collection of the resulting valuable products.
Pyrolysis is best understood not as a single event, but as a controlled, three-part transformation. It systematically deconstructs a feedstock into three valuable and distinct product streams: a solid (biochar), a liquid (bio-oil), and a gas (syngas).

Stage 1: Feedstock Drying
The Goal: Removing Moisture
The first stage prepares the raw material, or feedstock, for the main reaction. Its primary goal is to remove water.
Moisture consumes a significant amount of energy to vaporize and can lower the quality of the final liquid products. Efficient drying is the first step toward an efficient process.
How It Works
The feedstock is heated to temperatures slightly above the boiling point of water, typically between 100-150°C. This drives off any free or bound water as steam before the material enters the main reaction chamber.
Stage 2: The Core Pyrolysis Reaction
The Goal: Thermal Decomposition
This is the central stage where the actual chemical breakdown occurs. The objective is to thermally crack the large organic polymers of the feedstock into smaller, more valuable molecules.
How It Works: Heat Without Oxygen
The dried feedstock is heated to much higher temperatures (typically 400-800°C or higher) in an oxygen-free environment.
The absence of oxygen is critical. It prevents the material from burning (combustion) and instead forces it to break apart, creating a mixture of volatile vapors and a solid, carbon-rich char.
The Chemical Transformation
This intense heat breaks the complex chemical bonds in materials like biomass or plastic. The result is a solid residue (biochar) and a hot stream of gaseous compounds (pyrolysis vapors).
Stage 3: Product Separation and Collection
The Goal: Capturing Value
The hot mixture of gases and vapors produced during pyrolysis must be separated to capture the valuable end products.
How It Works: Cooling and Condensation
This vapor stream is rapidly cooled. As it cools, a significant portion of the gases condense into a liquid, which is then collected.
The Three Final Product Streams
This separation process yields the three signature products of pyrolysis:
- Solid (Biochar): A stable, carbon-rich solid similar to charcoal. It is often used in agriculture to improve soil health, as a filter (sorbent), or as a solid fuel.
- Liquid (Bio-oil): A dark, dense liquid formed from the condensed vapors. It can be used as an industrial fuel or further refined into transportation fuels and specialty chemicals.
- Gas (Syngas): The non-condensable portion of the vapors. This gas is rich in hydrogen and carbon monoxide and has a high energy content. It is almost always recycled on-site to provide the heat needed to power the entire pyrolysis process, making the system highly energy-efficient.
Understanding the Trade-offs
The ratio of these three products is not fixed. It can be deliberately manipulated by controlling the process conditions, representing a key trade-off in system design.
The Impact of Temperature and Heating Rate
The speed and temperature of the reaction are the most critical variables for determining the final product yields.
- Slow Pyrolysis: Lower temperatures and longer reaction times favor the production of biochar. This is the principle behind traditional charcoal making.
- Fast Pyrolysis: Very high temperatures and extremely short reaction times (often less than two seconds) are used to maximize the yield of bio-oil.
The Challenge of Feedstock
Different feedstocks produce different results. Wood, agricultural waste, and plastics will all break down into varying qualities and quantities of char, oil, and gas, requiring process adjustments.
The Role of the Reactor
The physical machinery used—such as a fixed-bed, fluidized-bed, or rotary kiln reactor—is chosen specifically to manage a certain feedstock and achieve a desired outcome, whether it's maximizing biochar or bio-oil.
Optimizing Pyrolysis for Your Goal
To apply this process effectively, you must first define your desired output. The operational parameters are then set to achieve that specific goal.
- If your primary focus is producing solid carbon (biochar): You should use a slow pyrolysis process with lower temperatures and longer material residence times.
- If your primary focus is generating liquid fuel (bio-oil): You must implement a fast pyrolysis process with rapid heating rates and efficient vapor quenching.
- If your primary focus is energy self-sufficiency: Your design must prioritize the efficient capture and combustion of the syngas to provide heat for the entire system.
By understanding these stages and their controlling variables, you can engineer the pyrolysis process to transform diverse feedstocks into valuable, targeted products.
Summary Table:
| Stage | Goal | Key Process | Typical Temperature |
|---|---|---|---|
| 1. Drying | Remove moisture from feedstock | Heating to 100-150°C to vaporize water | 100-150°C |
| 2. Pyrolysis Reaction | Thermally decompose material | Heating in oxygen-free environment (400-800°C+) | 400-800°C+ |
| 3. Product Collection | Separate and capture final products | Cooling and condensing vapors into bio-oil, biochar, syngas | Varies |
Ready to optimize your pyrolysis process? KINTEK specializes in laboratory equipment and consumables for pyrolysis research and development. Whether you're focused on maximizing biochar production, optimizing bio-oil yields, or achieving energy self-sufficiency with syngas, our expertise and high-quality equipment can help you achieve precise control over all three stages of pyrolysis. Contact our experts today to discuss how we can support your specific laboratory needs and help you transform diverse feedstocks into valuable, targeted products.
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What are the advantages of using an alumina liner in a tube furnace for biomass combustion corrosion simulations?
- How do you clean a tube furnace tube? A Step-by-Step Guide to Safe and Effective Cleaning
- What is the pressure on a tube furnace? Essential Safety Limits for Your Lab
- What tube is used for tubular furnace? Choose the Right Material for Temperature & Atmosphere
- What are the common applications for a tube furnace? Essential for Heat Treatment, Synthesis, and Purification



















