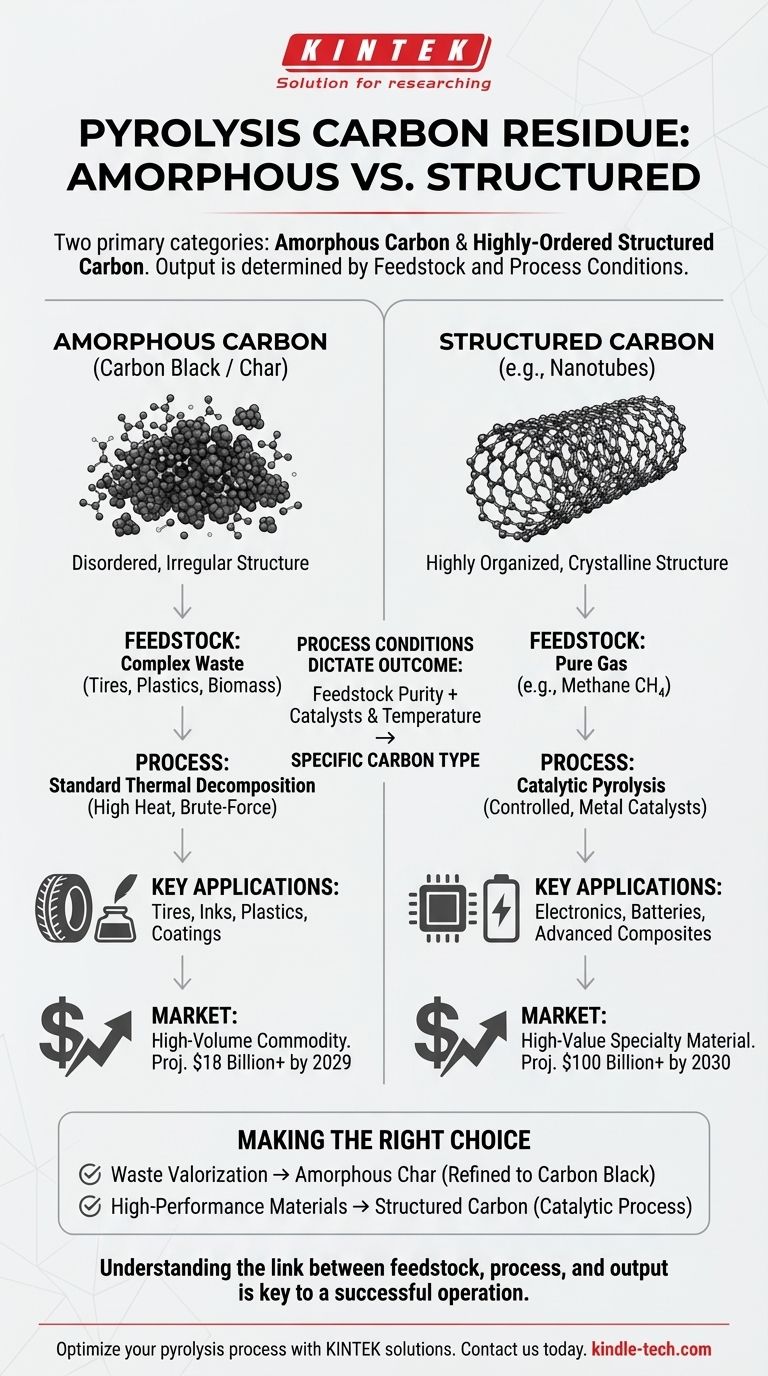
In pyrolysis, the two primary categories of solid carbon residue produced are amorphous carbon, commonly known as carbon black or char, and highly-ordered structured carbons, such as carbon nanotubes. The specific type of carbon generated is not accidental; it is determined by the input material (feedstock) and the precise conditions of the pyrolysis process, such as temperature and the presence of catalysts.
The critical distinction to grasp is that pyrolysis can either produce a lower-value, high-volume commodity (carbon black) from complex waste or a high-value, specialized material (carbon nanotubes) from pure feedstocks. This choice defines the entire economic and technical approach of the operation.

The Core Difference: Atomic Structure
The value and application of the final carbon product are dictated by how the carbon atoms arrange themselves during the process. This fundamental difference in structure creates two distinct classes of materials.
Amorphous Carbon (Carbon Black / Char)
Amorphous carbon has a disordered, irregular atomic structure. Think of it as a jumbled pile of carbon atoms with no long-range pattern.
This is the most common solid product from the pyrolysis of complex, heterogeneous feedstocks like waste tires, plastics, or biomass. It is often referred to as char or pyrolysis-derived carbon black.
Structured Carbon (e.g., Carbon Nanotubes)
Structured carbons, like carbon nanotubes, have a highly organized, crystalline structure. Carbon atoms are bonded together in a repeating, predictable pattern, forming materials with exceptional properties.
Producing these materials requires a more controlled and sophisticated process, typically involving the pyrolysis of a simple, pure gas feedstock like methane in the presence of a specific metal catalyst.
How Process Conditions Dictate the Outcome
You cannot get both types of carbon from the same process simultaneously. The output is a direct consequence of the engineering decisions made upstream.
The Role of Feedstock
The purity and composition of your input material are the single most important factors.
Complex materials like mixed plastics or old tires contain a wide variety of chemical compounds and impurities. Their thermal decomposition results in a similarly complex and disordered solid: amorphous char.
To create an ordered structure like a nanotube, you must start with a simple, uniform building block. A pure gas like methane (CH₄) provides a clean and consistent source of carbon atoms that can be guided into a specific structure.
The Influence of Catalysts and Temperature
Standard pyrolysis for waste processing simply uses high heat to break down materials. It is a brute-force thermal decomposition.
Creating structured carbons requires catalytic pyrolysis. In this process, specific metal catalysts (e.g., nickel, iron, cobalt) are introduced. At high temperatures, these catalyst particles act as "seeds" upon which the carbon atoms from the gas feedstock assemble into an ordered, graphitic structure.
Understanding the Value and Trade-offs
The economic models for producing these two types of carbon are vastly different, reflecting their distinct markets and applications.
Carbon Black: A High-Volume Commodity
Pyrolysis-derived carbon black is a valuable industrial commodity. The global market is substantial, projected to reach over $18 billion by 2029.
Its primary uses are as a reinforcing agent in tires and a pigment in inks, plastics, and coatings. However, char from waste pyrolysis often requires significant post-processing and purification to meet the quality standards for these applications, adding cost and complexity.
Carbon Nanotubes: A High-Value Specialty Material
Carbon nanotubes are advanced materials, not commodities. Their exceptional strength and electrical conductivity make them ideal for high-tech applications in electronics, batteries, and advanced composites.
Reflecting this high performance, the market for these materials is projected to exceed $100 billion by 2030. Production is a precision chemical engineering process, demanding higher capital investment and operational control than a typical waste pyrolysis plant.
Making the Right Choice for Your Goal
The type of carbon you aim to produce should be a deliberate decision based on your primary objective, whether it's waste management or advanced material manufacturing.
- If your primary focus is waste valorization (e.g., recycling tires or plastics): Your process will yield an amorphous carbon char, which can be refined into a marketable carbon black product.
- If your primary focus is producing high-performance materials for electronics: You must use a catalytic pyrolysis process with a pure gas feedstock to generate structured carbons like nanotubes.
Understanding this fundamental link between your feedstock, process, and carbon output is the key to designing a successful and economically viable pyrolysis operation.
Summary Table:
| Carbon Type | Feedstock | Process | Key Applications |
|---|---|---|---|
| Amorphous Carbon (Char) | Complex waste (tires, plastics, biomass) | Standard thermal decomposition | Tires, inks, plastics, coatings |
| Structured Carbon (e.g., Nanotubes) | Pure gas (e.g., methane) | Catalytic pyrolysis | Electronics, batteries, advanced composites |
Ready to optimize your pyrolysis process for high-value carbon production? KINTEK specializes in providing advanced lab equipment and consumables for precise pyrolysis applications. Whether you're exploring waste valorization or developing next-generation materials like carbon nanotubes, our solutions deliver the accuracy and reliability your lab needs. Contact our experts today to discuss how we can support your research and production goals.
Visual Guide
Related Products
- Graphite Vacuum Continuous Graphitization Furnace
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
People Also Ask
- Is graphite good in high temperature? Unlocking Its Extreme Heat Potential
- At what temperature does graphite thermal decompose? The Critical Role of Atmosphere
- What is the thermal conductivity of graphite at high temperatures? A Guide to Thermal Management in Extreme Heat
- What is the temperature resistance of graphite? Unlocking Its High-Temp Potential in Your Lab
- What is the graphite furnace used for? Achieve Extreme Heat Up to 3000°C in a Controlled Environment



















