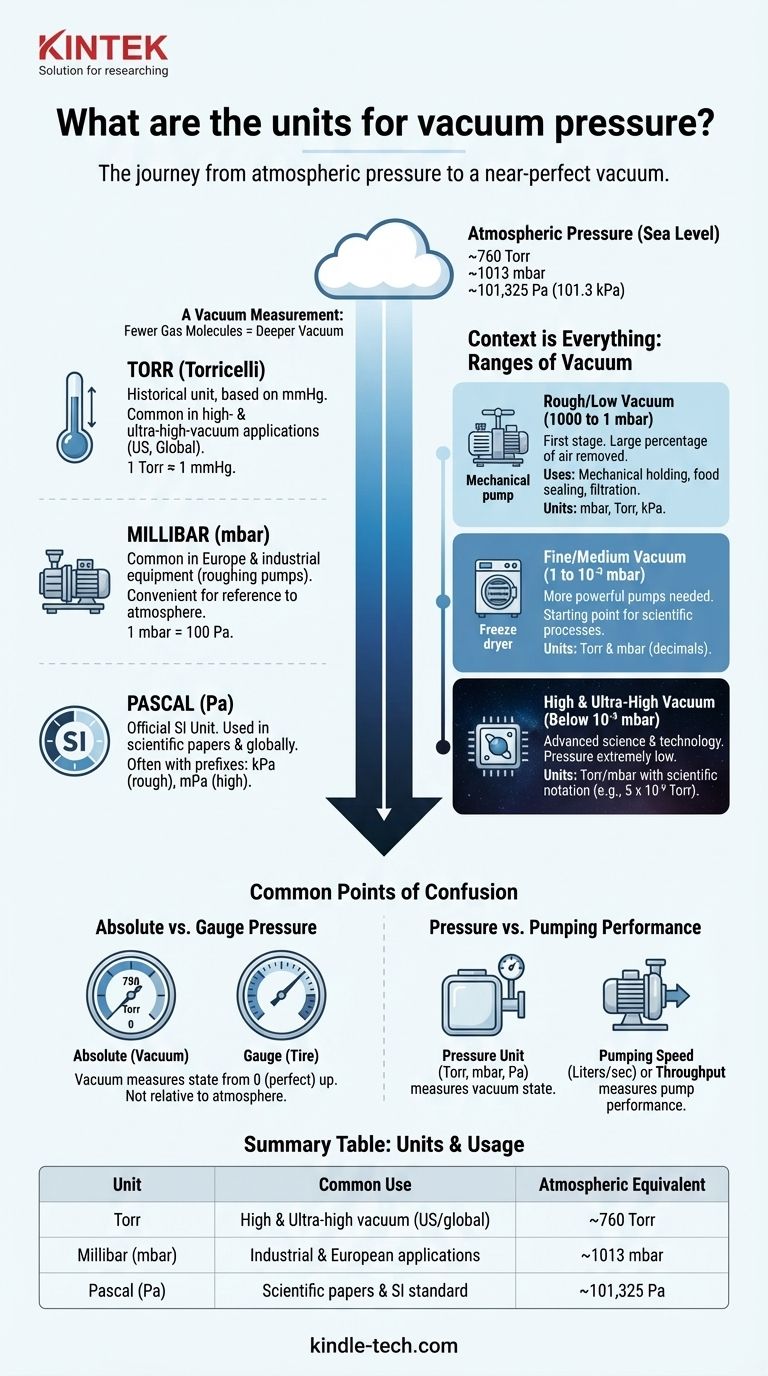
The most common units for vacuum pressure are Torr, millibar (mbar), and the official SI unit, the Pascal (Pa). Different industries and regions tend to favor one over the others, but all are used to measure pressure below the surrounding atmospheric pressure. A vacuum measurement describes how many gas molecules remain in a given volume; the lower the pressure reading, the fewer molecules are left and the "deeper" the vacuum.
Understanding vacuum pressure is less about memorizing unit conversions and more about knowing where your measurement falls on the scale from atmospheric pressure down to a near-perfect vacuum. The unit is simply the language used to describe a specific level of emptiness.
From Atmosphere to Emptiness: A Scale of Pressure
To understand vacuum units, you must first have a clear reference point: standard atmospheric pressure. All vacuum measurements are a journey downward from this starting line toward absolute zero pressure.
The Starting Point: Atmospheric Pressure
A vacuum is any pressure below the local atmospheric pressure. At sea level, this is standardized as 1 atmosphere (atm), which is equivalent to approximately 760 Torr, 1013 mbar, or 101,325 Pascals (101.3 kPa).
Torr: The High-Vacuum Standard
The Torr is a unit historically based on millimeters of mercury (mmHg) and is named after Evangelista Torricelli. One Torr is almost exactly equal to 1 mmHg. It is widely used in the United States and in high- and ultra-high-vacuum applications globally because of its convenient scale in those ranges.
Millibar (mbar): The European and Industrial Unit
The millibar (mbar) is common in Europe and for industrial vacuum equipment like roughing pumps. One millibar is equal to 100 Pascals. Because standard atmospheric pressure is about 1013 mbar, it provides a convenient reference where 1000 mbar is roughly one atmosphere.
Pascal (Pa): The Official SI Unit
As the official international standard unit for pressure, the Pascal (Pa) is used in many scientific papers and is the preferred unit in many countries outside the US. Due to its small magnitude, it is almost always used with a prefix, such as kiloPascal (kPa) for rough vacuum or milliPascal (mPa) for high vacuum.
Context is Everything: The Ranges of Vacuum
The "quality" of a vacuum is defined by its pressure range. Different units may be more common depending on the range you are working in.
Rough/Low Vacuum (1000 to 1 mbar)
This is the first and easiest level of vacuum to achieve, where a large percentage of air molecules are removed. It's used for processes like mechanical holding, food sealing, and filtration. All units (mbar, Torr, kPa) are commonly seen here.
Fine/Medium Vacuum (1 to 10⁻³ mbar)
This range requires more powerful pumps and is the starting point for many scientific processes like freeze-drying or distillation. Here, you begin to see Torr and mbar become the dominant units, often expressed in decimals.
High and Ultra-High Vacuum (Below 10⁻³ mbar)
This is the domain of advanced science and technology, such as semiconductor manufacturing, particle accelerators, and surface science. In these regimes, the pressure is so low that Torr or mbar combined with scientific notation (e.g., 5 x 10⁻⁹ Torr) is the standard language.
Common Points of Confusion
Navigating vacuum measurement involves more than just units. Understanding these key distinctions is critical for accurate work.
Absolute vs. Gauge Pressure
Vacuum is an absolute pressure measurement. Its scale starts at 0 (a perfect, theoretical vacuum) and goes up. This is different from gauge pressure (like tire pressure), which measures pressure relative to the surrounding atmosphere. A vacuum reading of 750 Torr is an absolute value, not "10 Torr below atmosphere."
Pressure vs. Pumping Performance
The unit of pressure (Torr, mbar, Pa) measures the state of the vacuum achieved inside a chamber. It is distinct from metrics that measure the performance of the vacuum pump itself, such as pumping speed (volume per time, e.g., Liters/sec) or throughput (mass flow rate).
Why So Many Units?
The variety of units is a result of historical development, regional preferences, and industry-specific conventions. An American physicist studying surface chemistry (using Torr) and a German engineer designing a packaging line (using mbar) are both measuring vacuum, just with different dialects.
Making the Right Choice for Your Goal
Your application will determine which units and ranges are most relevant to you.
- If your primary focus is general industrial or laboratory work: You will likely encounter Torr and mbar, and understanding the rough vs. fine vacuum distinction is most important.
- If your primary focus is HVAC or refrigeration: You may work with microns (a milliTorr) or inches of mercury (inHg) to ensure systems are free of moisture and non-condensable gases.
- If your primary focus is high-tech research or manufacturing: You will primarily use Torr or mbar with scientific notation to quantify conditions in the high and ultra-high vacuum ranges.
Ultimately, mastering vacuum measurement is about understanding your position on the vast scale from air to emptiness, not just converting between units.
Summary Table:
| Unit | Common Use | Atmospheric Pressure Equivalent |
|---|---|---|
| Torr | High & ultra-high vacuum (US/global) | ~760 Torr |
| Millibar (mbar) | Industrial & European applications | ~1013 mbar |
| Pascal (Pa) | Scientific papers & SI standard | ~101,325 Pa |
Need precise vacuum control for your lab? KINTEK specializes in lab equipment and consumables, providing reliable vacuum solutions tailored to your specific pressure range and application—whether you're working in rough vacuum or ultra-high vacuum. Contact our experts today to discuss how we can enhance your laboratory's efficiency and accuracy!
Visual Guide
Related Products
- Laboratory Vertical Water Circulating Vacuum Pump for Lab Use
- Laboratory Benchtop Water Circulating Vacuum Pump for Lab Use
- Ultra-Vacuum Electrode Feedthrough Connector Flange Power Electrode Lead for High-Precision Applications
- CF KF Flange Vacuum Electrode Feedthrough Lead Sealing Assembly for Vacuum Systems
- KF ISO Stainless Steel Vacuum Flange Blind Plate for High Vacuum Systems
People Also Ask
- What are the failures in a hydraulic system? Prevent Costly Downtime with Expert Diagnosis
- What happens if a hydraulic system leaks? Prevent Costly Damage and Safety Hazards
- What are the advantages of a water circulating vacuum pump? Superior Durability for Demanding Lab Environments
- What are the 5 factors that affect the rate of evaporation? Master the Process for Your Lab
- What were the 4 factors that affect the rate of evaporation? Master Control for Lab & Industrial Processes



















