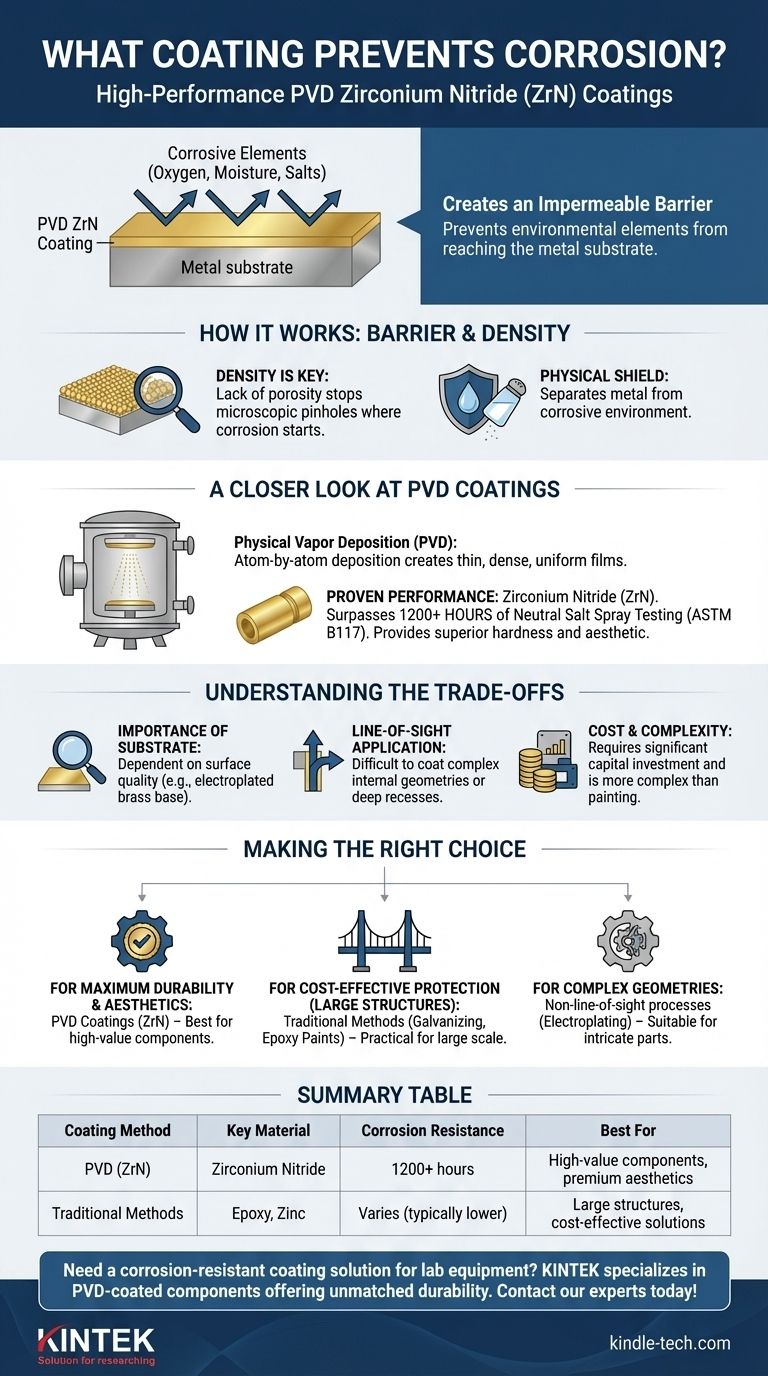
To prevent corrosion, high-performance coatings like Zirconium Nitride (ZrN), applied via Physical Vapor Deposition (PVD), are an excellent choice. This method creates an extremely dense and durable barrier on a material's surface. As a benchmark, PVD ZrN has been proven to withstand over 1200 hours of aggressive salt spray testing, far exceeding many industry requirements for corrosion resistance.
The most effective corrosion prevention is not just a single material, but a strategy. The goal is to create a complete, non-porous barrier that physically separates the underlying metal from its corrosive environment, and PVD is a highly advanced method for achieving this.

How Coatings Fundamentally Stop Corrosion
Corrosion is an electrochemical process where a refined metal attempts to revert to a more stable chemical state, like an ore. A coating's job is to interrupt this process.
Creating an Impermeable Barrier
The primary function of a corrosion-resistant coating is to act as a physical shield. It blocks environmental elements like oxygen, moisture, and salts from ever making contact with the metal substrate.
The Critical Role of Density
The effectiveness of this barrier depends entirely on the coating's density and its lack of porosity. Even microscopic pinholes can become initiation sites for corrosion, which can then spread underneath the coating, causing it to fail.
A Closer Look at PVD Coatings
Physical Vapor Deposition (PVD) is a family of processes used to produce high-performance thin films, offering superior corrosion resistance compared to many traditional methods.
What is Physical Vapor Deposition (PVD)?
PVD is a vacuum deposition method where a solid material is vaporized in a vacuum chamber and deposited onto a target object. This atom-by-atom deposition process creates an exceptionally thin, yet dense and strongly bonded, film.
Proven Performance: Zirconium Nitride (ZrN)
Zirconium Nitride (ZrN) is a specific type of PVD coating known for its gold-like appearance, hardness, and superb corrosion resistance. On substrates like electroplated brass, it has been shown to surpass 1200 hours of neutral salt spray testing (ASTM B117), a standard industry measure of corrosion performance.
Why PVD is So Effective
The PVD process results in a film structure that is highly uniform and virtually free of the porosity found in many other coating types. This density is the key reason it provides such a robust barrier against corrosive agents.
Understanding the Trade-offs
While highly effective, PVD is not a universal solution. Understanding its limitations is crucial for making an informed decision.
The Importance of the Substrate
The performance of any PVD coating is highly dependent on the quality of the surface it is applied to. The reference to "electroplated brass" is key; the underlying electroplating provides a smooth, compatible, and corrosion-resistant base layer that enhances the performance of the final PVD film. Improper surface preparation will cause any advanced coating to fail.
Line-of-Sight Application
PVD is a "line-of-sight" process. The vaporized material travels in a straight line to the substrate, making it difficult to uniformly coat complex internal geometries or deeply recessed areas.
Cost and Process Complexity
PVD requires significant capital investment in vacuum chambers and related equipment. It is a more complex and often more expensive process upfront compared to simpler methods like industrial painting, powder coating, or galvanizing.
Making the Right Choice for Your Application
Selecting the correct anti-corrosion strategy depends entirely on your product's requirements, environment, and budget.
- If your primary focus is maximum durability and a premium aesthetic: PVD coatings like ZrN are an industry-leading choice, especially for high-value components that must resist wear and environmental attack.
- If your primary focus is cost-effective protection for large, simple structures: Traditional methods like hot-dip galvanizing or specialized epoxy paints may be a more practical and economical solution.
- If your primary focus is coating parts with complex internal geometries: Processes that are not line-of-sight, such as electroplating or chemical conversion coatings, might be more suitable.
Choosing the right coating is about matching the technology's strengths to your specific operational goal.
Summary Table:
| Coating Method | Key Material | Corrosion Resistance (Salt Spray Test) | Best For |
|---|---|---|---|
| PVD (Physical Vapor Deposition) | Zirconium Nitride (ZrN) | 1200+ hours | High-value components, premium aesthetics |
| Traditional Methods (e.g., Painting, Galvanizing) | Epoxy, Zinc | Varies (typically lower than PVD) | Large structures, cost-effective solutions |
Need a corrosion-resistant coating solution tailored to your lab equipment? KINTEK specializes in high-performance lab equipment and consumables, including PVD-coated components that offer unmatched durability and protection. Our expertise ensures your laboratory tools withstand harsh environments, enhancing longevity and reliability. Contact our experts today to discuss how we can safeguard your investments with advanced coating technologies!
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- CVD Diamond Domes for Industrial and Scientific Applications
- Laboratory CVD Boron Doped Diamond Materials
- Custom PTFE Teflon Parts Manufacturer Corrosion Resistant Cleaning Rack Flower Basket
- Vacuum Hot Press Furnace Machine for Lamination and Heating
People Also Ask
- What is the deposition rate of CVD? A Key Advantage for Efficient Thin-Film Manufacturing
- What are the disadvantages of ion beam deposition? High Precision at the Cost of Speed and Scalability
- What role does Chemical Vapor Deposition (CVD) equipment play in the preparation of C/C composites? Expert Analysis
- What are the chemical vapour deposition instruments? A Guide to CVD, PECVD & ICPCVD Systems
- What is chemical vapor deposition method of nanomaterials? Build Atom-by-Atom with Precise Control
- What are the advantages of CVD for lithium anodes? Enhance Battery Stability with Precision Thin-Film Protection
- How does the heating of the substrate influence the quality of titanium carbide films? Optimize CVD Coating Performance
- What are the primary advantages of utilizing a horizontal hot-wall CVD reactor? Gain Industrial Alumina Coating Quality



















