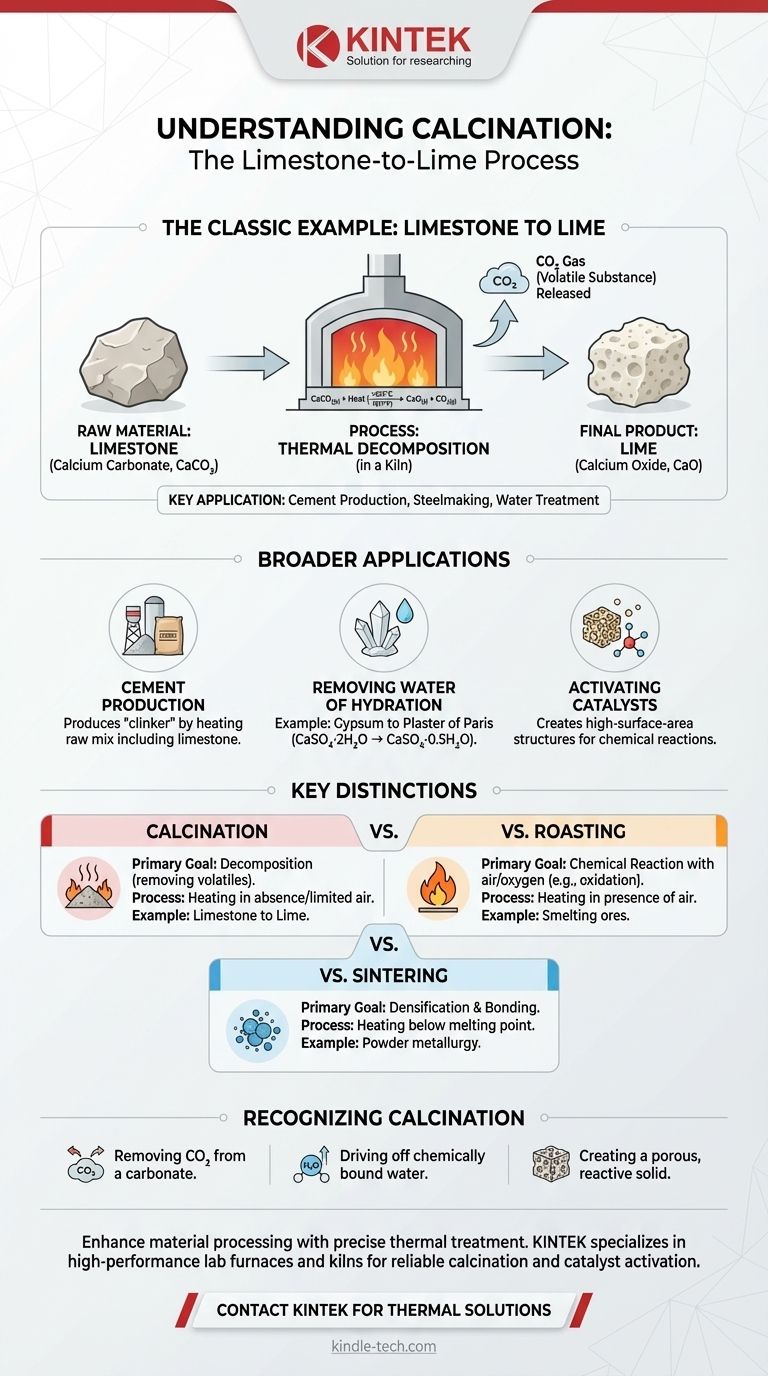
The most classic example of calcination is the industrial production of lime from limestone. In this process, limestone (calcium carbonate, CaCO₃) is heated to a high temperature inside a kiln, causing it to decompose. This thermal treatment drives off carbon dioxide (CO₂) gas, leaving behind a chemically distinct and commercially valuable product known as lime (calcium oxide, CaO).
Calcination is not simply heating; it is a precise thermal decomposition process. Its core purpose is to remove volatile substances, like carbon dioxide or water, from a solid to fundamentally alter its chemical and physical properties.

The Limestone-to-Lime Process Explained
Understanding the conversion of limestone to lime provides a perfect window into the principles of calcination. This process is one of the oldest chemical transformations used by humanity and remains a cornerstone of modern industry.
The Raw Material: Limestone
Limestone is a sedimentary rock composed primarily of calcium carbonate (CaCO₃). It is an abundant, low-cost raw material quarried directly from the earth.
The Mechanism: Thermal Decomposition
When heated to a sufficiently high temperature—typically above 825°C (1517°F)—the calcium carbonate in limestone becomes unstable. The heat provides the energy needed to break the chemical bonds, initiating a decomposition reaction.
The simple chemical equation for this is: CaCO₃(s) + Heat → CaO(s) + CO₂(g)
This shows the solid limestone breaking down into solid lime (calcium oxide) and carbon dioxide gas.
The Equipment: The Kiln
This transformation is conducted in a specialized high-temperature oven called a kiln or furnace. Industrial kilns, such as rotary or shaft kilns, are designed to heat the limestone uniformly and allow the released CO₂ gas to be safely removed.
The Final Product: Lime
The resulting solid, calcium oxide (CaO), is often called quicklime. It is physically and chemically different from the original limestone. It is typically more porous, more reactive, and has a lower density.
Why Calcination Matters: Broader Applications
While the limestone example is foundational, calcination is a critical step in many other industrial processes. The underlying principle of removing volatile components through heat is widely applied.
Producing Cement
The manufacturing of Portland cement, the most common type of cement, relies heavily on calcination. A raw mix including limestone and clay is heated in a kiln to produce "clinker," an intermediate product. The calcination of the limestone within this mix is a crucial first step.
Removing Water of Hydration
Calcination is also used to remove chemically bound water (water of hydration) from minerals. For example, heating gypsum (CaSO₄·2H₂O) drives off water to produce plaster of Paris (CaSO₄·0.5H₂O). A similar process is used on bauxite ore as part of aluminum production.
Activating Catalysts
In chemical manufacturing, some materials must be "activated" before they can function as catalysts. Calcination is often used to heat catalyst precursors to create a final porous and high-surface-area structure ideal for promoting chemical reactions.
Key Distinctions to Understand
To truly grasp the concept, it's important to distinguish calcination from other thermal processes that might seem similar.
Calcination vs. Roasting
Roasting is a process that involves heating a material in the presence of air or oxygen to induce a chemical reaction with it, such as oxidation. Smelting metallic ores is a common example. In contrast, calcination is primarily about decomposition due to heat itself, not a reaction with the surrounding air.
Calcination vs. Sintering
Sintering involves heating a powdered material to a temperature below its melting point to cause its particles to adhere and bond, increasing its strength and density. While calcination might precede sintering, its goal is decomposition, not densification.
Recognizing Calcination in Practice
To identify this process, focus on the fundamental goal and outcome.
- If the primary focus is removing CO₂ from a carbonate: This is the most definitive form of calcination, as seen with limestone.
- If the primary focus is driving off chemically bound water from a hydrated mineral: This is another major application of calcination, used to create materials like plaster or prepare alumina.
- If the primary focus is creating a more porous, reactive solid by removing a volatile component: This change in physical properties is a key signature of a successful calcination process.
Ultimately, calcination is a fundamental tool used across industries to transform raw materials into more useful substances by precisely removing a part of their chemical structure.
Summary Table:
| Aspect | Details for Limestone Example |
|---|---|
| Raw Material | Limestone (Calcium Carbonate, CaCO₃) |
| Process | Thermal Decomposition |
| Temperature | > 825°C (1517°F) |
| Equipment | Kiln (e.g., Rotary, Shaft) |
| Reaction | CaCO₃(s) + Heat → CaO(s) + CO₂(g) |
| Product | Lime (Calcium Oxide, CaO) |
| Key Application | Cement Production, Steelmaking, Water Treatment |
Ready to enhance your material processing with precise thermal treatment?
At KINTEK, we specialize in high-performance lab furnaces and kilns designed for reliable calcination processes, catalyst activation, and more. Whether you are in R&D or quality control, our equipment helps you achieve consistent, high-purity results.
Let's discuss your application needs. Contact our experts today to find the perfect thermal solution for your laboratory.
Visual Guide
Related Products
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Disc Rotary Mixer for Efficient Sample Mixing and Homogenization
People Also Ask
- What are the zones in rotary kiln in cement production? Master the Core Process for High-Quality Clinker
- What is the purpose of a calciner? Boost Efficiency in High-Temperature Processing
- What is the principle of rotary kiln? Mastering Continuous Thermal Processing
- What are the different types of reactors in plastic pyrolysis? Choose the Right System for Your Waste
- What are the industrial applications of pyrolysis? Transform Waste into Energy and Valuable Products



















