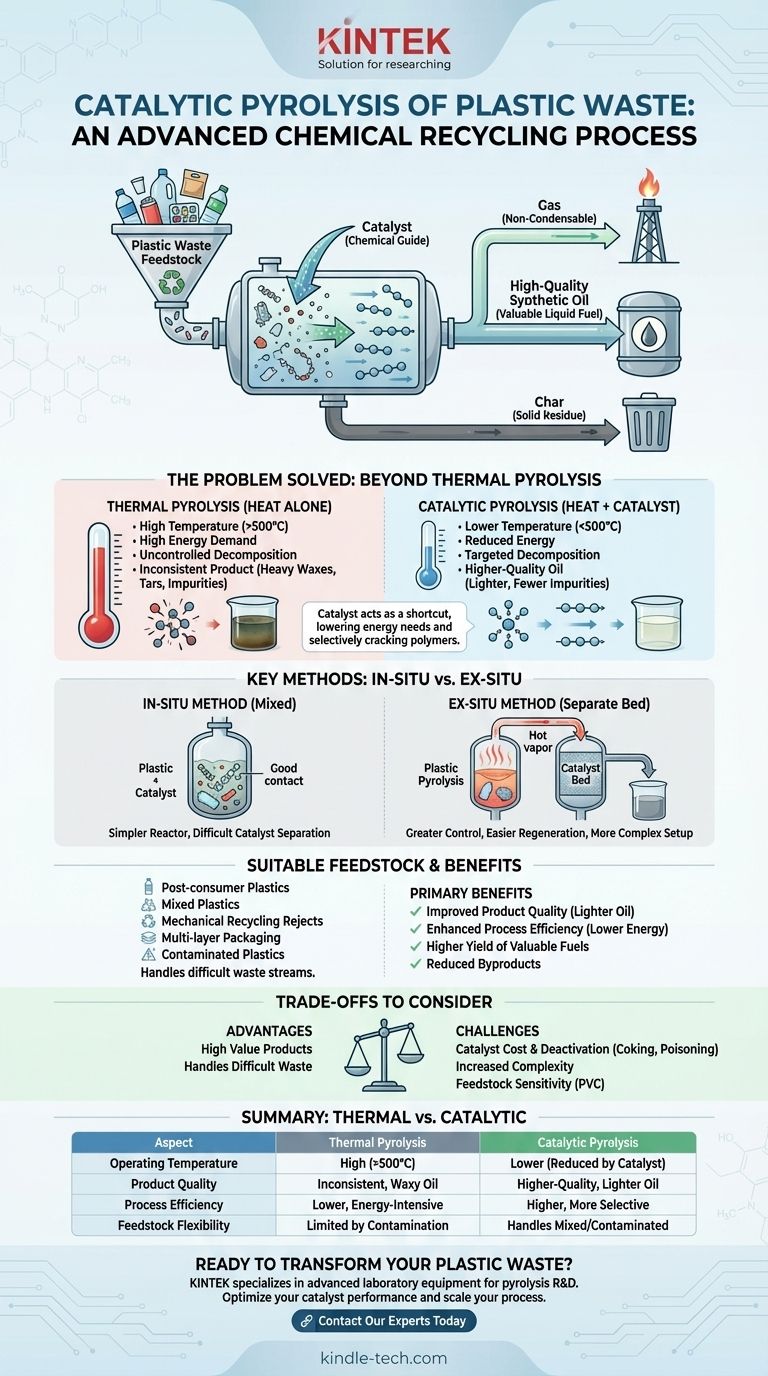
In essence, catalytic pyrolysis of plastic waste is an advanced chemical recycling process that uses a substance called a catalyst to break down complex plastic polymers into simpler, valuable products like synthetic oil, gas, and char. Unlike traditional pyrolysis, the catalyst actively steers the chemical reactions, improving the efficiency of the process and the quality of the output.
The core purpose of using a catalyst is not just to break down plastic waste, but to do so with greater control. It allows for lower operating temperatures and selectively guides the decomposition to yield a higher-quality, more consistent synthetic oil, addressing the core challenge of inconsistent plastic feedstock.

The Problem Catalytic Pyrolysis Solves
To understand the value of catalytic pyrolysis, we must first recognize the limitations of breaking down plastics with heat alone (thermal pyrolysis).
The Limits of Heat Alone
Standard thermal pyrolysis requires very high temperatures (typically above 500°C) to break the strong chemical bonds in plastics. This high energy demand makes the process expensive.
Furthermore, without any guidance, the plastics break down into a wide, unpredictable range of products, including low-value heavy waxes, tars, and a high percentage of non-condensable gases. The resulting synthetic oil is often inconsistent and requires significant downstream purification.
The Catalyst as a Chemical "Guide"
A catalyst acts as a chemical shortcut, lowering the amount of energy (temperature) needed to initiate and sustain the plastic decomposition. It provides an alternative reaction pathway that is more efficient.
More importantly, specific catalysts are chosen for their ability to selectively "crack" the long plastic polymer chains into more desirable, shorter-chain hydrocarbons. This results in a synthetic oil that is lighter, more similar to conventional diesel or gasoline fractions, and has fewer impurities.
Primary Benefits of Using a Catalyst
The introduction of a catalyst delivers two critical advantages: improved product quality and enhanced process efficiency.
By guiding the chemical reactions, the process yields a higher proportion of valuable liquid fuel and reduces the production of unwanted byproducts like heavy wax and char.
This targeted approach also means the process can run at lower temperatures and often at faster rates, significantly reducing the overall energy consumption and operational cost compared to traditional thermal pyrolysis.
How Catalytic Pyrolysis Works: Key Methods
There are two primary configurations for introducing the catalyst into the pyrolysis system, each with its own operational profile.
The In-Situ Method: Catalyst and Plastic Mixed
In an in-situ (or "in-place") process, the catalyst is directly mixed with the shredded plastic feedstock inside the main pyrolysis reactor.
This approach benefits from a simpler reactor design and ensures excellent contact between the catalyst and the decomposing plastic vapors. However, separating the spent catalyst from the resulting solid char for reuse can be difficult.
The Ex-Situ Method: Separate Catalyst Bed
In an ex-situ (or "out-of-place") process, the pyrolysis occurs in one reactor, and the resulting hot vapor is then passed through a second, separate reactor containing the catalyst bed.
This dual-bed system offers far greater control. It allows for easier regeneration or replacement of the catalyst without interrupting the entire process and prevents the catalyst from being contaminated by inorganic materials in the feedstock. The trade-off is a more complex and capital-intensive setup.
Suitable Feedstock for Catalytic Pyrolysis
A significant advantage of this technology is its ability to handle waste streams that are difficult or impossible to process through mechanical recycling.
Suitable materials include:
- Post-consumer plastics: Everyday plastic packaging and items.
- Mixed plastics: Unsorted bales of different plastic types from municipal solid waste.
- Mechanical recycling rejects: The residual plastic that recyclers cannot process.
- Multi-layer packaging: Complex films like food pouches made of bonded layers of different materials.
- Contaminated plastics: Feedstocks containing impurities, including some levels of PET/PVC.
Understanding the Trade-offs
While powerful, catalytic pyrolysis is not without its challenges. A clear understanding of the trade-offs is essential for any practical application.
Catalyst Cost and Deactivation
Catalysts, especially highly selective ones, can be expensive. Over time, they lose their effectiveness (deactivate) due to carbon deposits (coking) or poisoning from contaminants in the plastic waste, such as chlorine from PVC. This necessitates periodic regeneration or replacement, adding to operational costs.
Increased Process Complexity
Implementing a catalytic system, particularly an ex-situ configuration, adds a layer of engineering complexity and requires more sophisticated process controls compared to a simple thermal pyrolysis unit.
Feedstock Sensitivity
While robust, catalysts are not immune to the contents of the waste stream. High concentrations of certain materials, like PVC, can rapidly poison the catalyst, reducing its lifespan and the process's overall effectiveness. Pre-treatment of feedstock may still be required to optimize performance.
Making the Right Choice for Your Goal
Catalytic pyrolysis represents a significant technological step toward creating a circular economy for plastic waste by converting it into a high-value resource.
- If your primary focus is maximizing the value of recovered resources: Catalytic pyrolysis is the superior choice, as it produces a higher-grade synthetic oil that requires less upgrading to become a final product.
- If your primary focus is processing difficult, mixed plastic waste: This technology's ability to handle contaminated and multi-layer plastics makes it a powerful solution for waste streams that have no other recycling outlet.
- If your primary focus is balancing performance with operational cost: The choice between in-situ and ex-situ methods depends on capital availability, with ex-situ offering better long-term control at a higher initial cost.
Ultimately, catalytic pyrolysis transforms the challenge of plastic waste into an opportunity for efficient and targeted resource recovery.
Summary Table:
| Aspect | Thermal Pyrolysis | Catalytic Pyrolysis |
|---|---|---|
| Operating Temperature | High (>500°C) | Lower (reduced by catalyst) |
| Product Quality | Inconsistent, waxy oil | Higher-quality, lighter oil |
| Process Efficiency | Lower, energy-intensive | Higher, more selective |
| Feedstock Flexibility | Limited by contamination | Handles mixed/contaminated plastics |
Ready to transform your plastic waste into valuable resources? KINTEK specializes in advanced laboratory equipment for pyrolysis research and development. Whether you're optimizing catalyst performance or scaling your process, our solutions help you achieve efficient, high-yield plastic conversion. Contact our experts today to discuss how we can support your catalytic pyrolysis projects with precision equipment and consumables tailored to your lab's needs.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
People Also Ask
- What are the functions of vacuum-sealing raw materials in a quartz tube during sulfide electrolyte synthesis?
- What is the primary purpose of air pre-oxidation in a tube furnace? Optimize Nano-Pd Catalyst Synthesis Today
- How does a high-temperature tube furnace facilitate the sintering and densification of Yttria-stabilized Ceria ceramics?
- Why is a high-temperature tube furnace combined with hydrogen reduction used for nickel powder? Achieve 99.90% Purity
- What role does a tube furnace play in the propane thermal cracking process? Key Functions for Ethylene Production
- What is the temperature of a quartz tube furnace? Master the Limits for Safe, High-Temp Operation
- What is the role of a cracking furnace? Transforming Hydrocarbons into Valuable Chemical Building Blocks
- What are ceramic tubes used for? Essential Components for Extreme Heat & Electrical Insulation



















